
Your choice of NEN™ radiochemicals
How do I know which formulation is appropriate for my application?
NEN radiochemicals are available in a variety of concentrations, specific activities, buffers, shipping, packaging and storage conditions. For help, see Which formulation should I choose?.
- Deoxyribonucleotide (DNA nucleotide) formulations
- Ribonucleotide (RNA nucleotide) formulations
- Nucleotide formulations for kinase and other protein-based assays
- 35S-Methionine formulations
- 3H-Thymidine formulations
- Iodine formulations for protein labeling
- Glucose formulations for glucose uptake assays
- Phosphate formulations for cell labeling
- Radioligands for ligand binding assays
How are NEN radiochemicals packaged?
Many NEN brand research products, including 32P-, 35S-, 3H-, 14C- and 125I-labeled compounds, are packaged in the NENSure System, as are most of our radiolabeled products. This system is easy to use and allows safe storage of unused product while also reducing radiation exposure.

Yellow plastic container for 35S products, black plastic container for 32P products and grey plastic lead lined container for nuclides.
Why not always ship in lead?
Lead is a hazardous material that cannot be readily disposed of and requires caution when handling. Although lead is a good radiation shield, it should only be used when dose rates are significantly high as required by regulatory agencies. Be assured NEN Radiochemicals are always packaged and shipped within regulations. For questions regarding regulations at your facility, consult with your local Radiation Safety Officer.
Receiving the shipment
Step-by-step unpacking, storage, and documentation
Radiochemicals decompose over time; therefore, upon receiving radioactive product, careful handling and proper storage is required. Every radioactive catalog product has a detailed Technical Data Sheet which typically contains information on the chemical structure (with labeled position), radiochemical purity, method of synthesis and recommendations on the best storage and handling methods for the specific product in question. The Technical Data Sheets are available online. Please read the Technical Data Sheet carefully before using and/or storing the product.
Before you order
- Schedule your purchase to avoid prolonged storage and use the product as soon as possible after taking delivery.
Receiving the shipment
- Upon receipt, inspect all packages to ensure that they have no damage or radioactive contamination. Perform a swipe test to make sure surfaces are within acceptable limits.
- Open the package in a designated area for radioactive work and wear protective gloves.
- Inspect the contents of the vial and verify the quantity and compound on the vial label and packing slip.
Aliquotting and storage
- When opening the vial, be aware of potential overpressure. Carefully open the vial in a fume-hood and monitor the surrounding area for contamination. See How to open a NENSure vial
- Avoid reopening of vials and freeze/thaw cycles. If a radiochemical is to be used at several experiments over a period of days, weeks, or months, it is advisable to aliquot it into several vials, keeping those to be used later under proper storage conditions until required.
- Many buffers and salts used in biological systems can be harmful to radiochemicals. Use clean pipet tips or syringes when withdrawing aliquots from the primary container.
- Radiochemicals in general should be kept in the dark as much as is practical. Particularly light-sensitive compounds are delivered in a UV-absorbing version of the NENSure vial. Handle these compounds under a red light and cover the primary container with foil during storage.
- If the compound is air- or water-sensitive, gently blow a stream of argon into the container before resealing it.
- Store at low temperatures, but do not freeze unless this is specifically recommended on the Technical Data Sheet.
- Store radiochemicals at the lowest usable specific activity. In general, a compound at high specific activity will decompose faster than one at a lower specific activity.
- When possible, dissolve the compound in a highly purified grade of solvent. Most common laboratory solvents have trace contaminants that will accelerate decomposition.
- Recheck the labeled compounds for purity at appropriate intervals.
How to open a NENSure vial

Figure 2. NENSure vial packaging system with description of the different components.
Using a pipette to remove the contents
To remove the cap
- Unscrew the lid of the outer container.
- Invert the lid and position it so that the built-in octagonal wrench engages the cap.
- Turn the wrench counterclockwise to remove cap.
To replace the cap
- Position the cap on the vial.
- Turn wrench clockwise to screw the cap in place, ensuring a tight seal.
Using a syringe to remove the contents
A 1.5-inch (38 mm) needle will reach the bottom of the V-insert when it is inserted directly through the septum of a 0.9 mL or 2.2 mL NENSure vial. A 2.25-inch (57 mm) needle is required for the 5 mL vial.
To expose the septum
- Unscrew the lid of the outer container.
- Slide aside the dust cover on the cap. If desired, remove the dust cover.
- If required install a charcoal trap (see figure and description below).
- Insert the syringe directly through the septum.
To use the needle guide
The needle guide was designed to facilitate the use of microsyringes, such as the Hamilton syringe. However, it will accommodate needles of 22 gauge or finer. When using the needle guide, add 0.5 inch (12.7 mm) to the required needle length.
- Slide aside and remove the dust cover.
- Insert and press the needle guide into the exposed opening.
- If required install a charcoal trap through the side opening of the guide until it penetrates the septum (see figure and instructions below).
- Fit the syringe needle into the center hole of the guide and push firmly through the septum into the vial.
Installing a charcoal trap
Prior to removing the vial contents, it is recommended to install a charcoal trap (NEX033T) to ventilate any volatiles that may have built up during shipment. This is especially important when opening volatile radioisotopes, such as 35S- or 125I-labeled compounds.
The charcoal trap can be installed either directly through the septum or through the side opening of the needle guide.
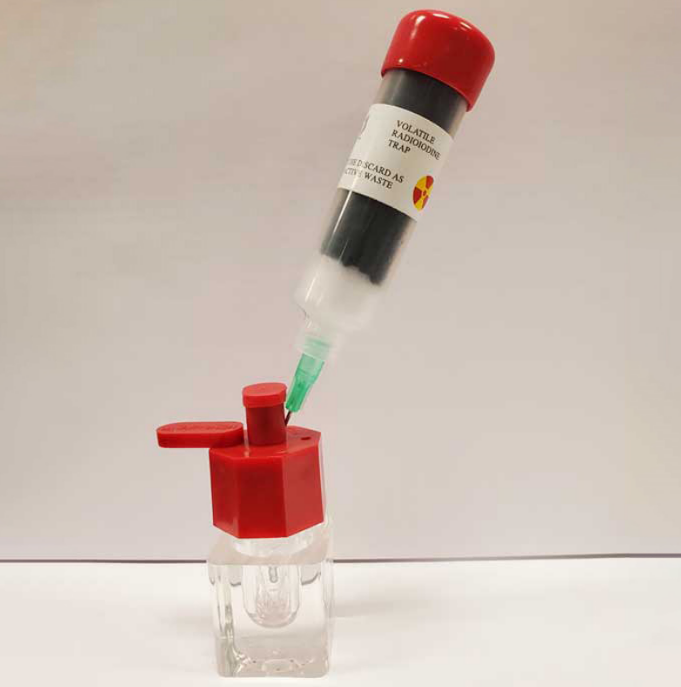
Figure 3. Charcoal Trap installed through the Needle Guide
Ensuring the longest shelf-life for your radiochemical
Compounds that are labeled with radioisotopes decompose faster than unlabeled counterparts. Although it is important to select an isotope based on its half-life and specific activity, it is the shelf-life (the time during which a labeled compound may be used with safety and confidence), that is of importance, both for the user of the compound and to the supplier of such compound.
How long a radiolabeled compound can be used depends on the modes by which the radiolabeled compound decomposes, and the methods used to control the stability. To help you get the best possible use from our radiochemicals, we have listed some recommendations that will help minimize the decomposition of radiochemicals and hence ensure that the radiochemical can be used well within its shelf-life.
- Store at the correct temperature - Upon arrival of the radiochemical at your facility, it is important that the material is stored at the temperature as indicated in the Technical Data Sheet. As a rule, compounds are best kept at low temperatures since chemical reactions are decelerated by a decrease in temperature; hence, the chemical stability of a radiochemical will be increased by lowering the temperature. However, please note that not all solutions of radiochemicals should be stored frozen. Some aqueous solutions that are frozen slowly, at say -20°C, can be susceptible to an effect called molecular clustering. This occurs because the solvent around the edge of the sample freezes first. The clustering of sample may result in an increased radiolytic decomposition and decrease in radiochemical stability. Therefore, freezing aqueous radiochemical solutions when not recommended should be avoided.
- Avoid reopening vials and freeze/thaw cycles - If you plan to use a radiochemical over a longer period, it is advisable to aliquot the product into several vials and keep those to be used later in the refrigerator or freezer until required.
- Protect from light - It is recommended to keep radiochemicals in the dark as much as is practical. Solutions of radiochemicals are especially vulnerable to chemical decomposition in the presence of light. Particularly light-sensitive radiolabeled compounds are supplied in dedicated NENSure vials containing an UV inhibitor, which protects the content from light.
- Protect from air - Certain radiochemicals are air-sensitive and therefore supplied under an inert gas, such as nitrogen or argon. Argon and nitrogen acts as a preservative. It is used to displace oxygen and humidity from the air in packed material to prolong the shelf-life. Oxygen and moisture will otherwise speed up the hydrolysis of the product. To sustain stability, it is advisable to blow a stream of inert gas before closing the caps of the aliquots.
- Purity - It is important that radiochemical compounds are of the highest purity in terms of radiochemical purity and as high as is practical in terms of chemical purity. However, an absolute purity of 100% cannot be achieved in practice. For all radiochemicals, the highest purity is obtained at the time of final purification. This is reported in the Technical Data Sheet in terms of percentage radiochemical purity and the methods used to measure the purity.
During storage, decomposition will reduce the purity. Therefore, it may be necessary to repurify a labeled compound to meet the quality required or to obtain a fresh supply.
Calculations for radiochemical products
Visit the Radiochemical calculations page for step-by-step help with the following calculations:
- Specific activity and radioactive concentration on the date of use
- Adjusting the specific activity
- Volume calculations for radiolabeled compounds
- Volume calculations for radionuclides
- Molarity of radiolabeled material
- Percent radioactive material
- Converting dpm to moles
Liquid scintillation counting
Click here for more information on liquid scintillation counting, sample preparation, cocktails, vials, plates, and detection instruments.
Applications
Scintillation proximity assays
Understanding radiochemical terminology and principles
Radioactivity is the property that some atoms, called radionuclides, have of undergoing spontaneous nuclear transformation. This process typically results in the emission of some form of radiation. The number of protons in an atom determines the element. For example, an atom with six protons in the nucleus is carbon whereas an atom having 8 protons in the nucleus is oxygen. Variants of an element that possess a greater or lesser number of neutrons are called isotopes. Some isotopes are stable – meaning that they do not spontaneously undergo nuclear transformation– but others are not. It is these unstable isotopes that we label as radioactive.
Isotopic properties
| Isotope | Half-Life | Maximum Theor. S/A | Energy Emitted |
|---|---|---|---|
| 32P | 14.3 Days | 9120 Ci/mmol | B-1.71MeV |
| 35S | 87.4 Days | 1488 Ci/mmol | B-0.167MeV |
| 3H | 12.28 Years | 29 Ci/mmol | B-0.019MeV |
| 125I | 60.0 Days | 2200 Ci/mmol | g-0.035MeV |
| 14C | 5,730 Years | 62 mCi/mmol | B-0.156MeV |
Table 1. Overview of some common isotope properties
Radioactive decay and half-life
Radioactive decay is the process by which an unstable isotope emits energy to reach a more stable state (entropy). Radioactive material decays until only stable substance is left and the decay of a substance is fixed and measurable.
Half-life is defined as the time it takes for a product to decay sufficiently to convert half of its mass to a stable form. The half-life of an isotope is fixed. Different half-lives affect the time limit of product use, for example:
- 32P can only be used for a few weeks
- 35S can sometimes last as long as 3-4 months
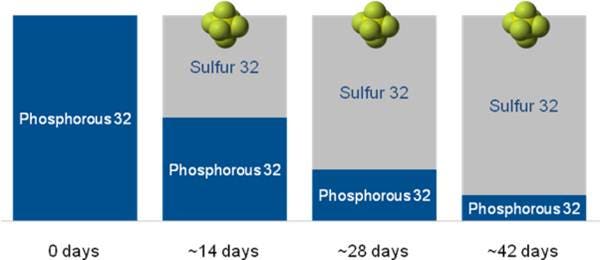
Figure. Simulated decay of 32P.
Radioactivity units - measured through decay
- Curie (Ci) The most commonly used unit of radioactivity, 1 Curie is defined as 3.7 X 1010 disintegration per second (dps)
- Becquerel (Bq) The IUPAC unit of radioactivity. 1 Becquerel is defined as 1 disintegration per second (dps)
- To convert from Bq to Ci and vice versa:
- 1 Bq = 2.7X10-11 Ci = 1 dps = 60 dpm
- 1 Ci = 3.7X1010 Bq = 3.7X1010 dps = 2.22X1012 dpm
- dpm — the abbreviation for disintegration per minute. This unit is directly related to the counts that one obtains in a radiometric detection instrument.
Specific activity
The specific activity is one of the key factors you need to determine which product to use for your detection method and to obtain optimal assay efficiency.
- Specific activity — the amount of radiolabeled mass in a sample, often expressed as Ci/mg or Ci/mmol
- Maximum specific activity — Each radioisotope has a maximum theoretical specific activity, often measured in Ci/mmol, Ci/mg or Ci/g. This is the specific activity if 100% of the molecules contain one isotopic label.
Concentration
Concentration refers to the amount of material (solute) dissolved in a given volume of liquid (solvent). There are two ways of expressing concentration when dealing with radioactivity:
- Radiochemical concentration (mCi/mL or µCi/mL) = total amount of radioactivity per unit volume
- Molar concentration (µM) = total molar amount of both the labeled and unlabeled product per unit volume
The radiochemical and molar concentration may dictate what you need to purchase.
- The radiochemical concentration and the molar concentration have a direct linear relationship.
The specific activity (SA) is the same in the products below, but the radiochemical and molar concentrations are different.
| 32P-UTP | SA (Ci/mmol) |
Radiochemical concentration (mCi/mL) |
Molar concentration |
|---|---|---|---|
| BLU507X | 800 Ci/mmol | 10 mCi/mL | 12.5 µM |
| BLU507T | 800 Ci/mmol | 20 mCi/mL | 25 µM |
| BLU507C | 800 Ci/mmol | 40 mCi/mL | 50 µM |
Radionuclide products and radiolabeled compounds - understanding the differences
Radionuclide products are products that consist of the radioisotope of a given element, usually in the form of a salt.
- Examples include 125Iodine in the form of sodium iodide (used for labeling proteins) and 51Chromium in the form of sodium chromate (used for cellular assays).
- Radionuclides are often provided as a salt, which will dissociate in solution to the radionuclide and its counterion (Example: 51Chromium chloride).
- Specific activity of radionuclides is expressed as Ci/g or Ci/mmol of the nuclide only (not including the mass of the counterion). If you want to convert from Ci/g to Ci/mmol or vice versa, you will use the molecular weight of the nuclide itself, not the full salt.
- When a radionuclide is supplied as "carrier-free" (with no cold material added), specific activity of the radionuclide does not change over time even though the amount of radioactivity decreases through decay. This is because the radionuclide becomes a different element after the decay event.
Radiolabeled compounds are compounds that are chemically bonded to a radioactive atom at one or more positions.
- Examples include 32P-ATP, 3H-Thymidine, 35S-Methionine, etc.
- Specific activity of radiolabeled compounds is expressed as Ci/mg or Ci/mmol of the compound (for example, Curies per millimole of ATP, or Curies per millimole of thymidine, etc.).
- Specific activity typically decreases over time for radiolabeled compounds as radioactive decay proceeds, unless the compound is purified in a manner that separates any unlabeled molecules prior to packaging. Some 125I-labeled radiochemicals are supplied at maximum specific activity (any unlabeled molecules have been separated out during purification). The specific activity of these products will not change over time, because the labeled molecules undergo catastrophic decay, and there should be no unlabeled material in the vial. Products that fall into this category will have this information mentioned on the technical data sheet, under "Specific Activity".
- The specific activity for a radiolabeled compound may sometimes appear higher than the theoretical maximum specific activity for a given radioisotope. This occurs when the compound is labeled at two or more positions.
Revvity nomenclature for radiochemicals
Revvity's radiochemical product offerings are listed by the full chemical name (salt forms are listed as the parent compound). Following the full chemical name is a bracket, separated from the name by a comma and space. The information within the bracket describes the location and type of radioactive label. The bracket is followed by a hyphen. Everything between the comma and space referenced above and the hyphen following the bracket would be placed before the chemical name or before the labeled unit to convert to the "square bracket preceding" system.
For example, Deoxy-D-glucose, 2-[1,2-3H (N)]-
The isotope employed is designated by its chemical symbol with the mass number shown as a superscript preceding the symbol.
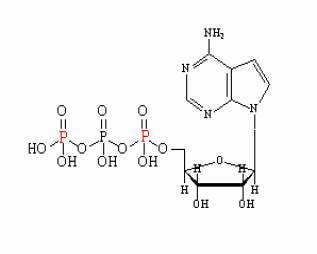
Figure. Structure of a 32P alpha/gamma labeled ATP. The phosphate (P) in red indicates the possible labeling positions.
- Specifically labeled compounds
This designation is used when all labeled positions are identified and the radioactivity at these positions is greater than 95% of the total incorporated into the compound. When more than one position is labeled, uniform or even distribution is not implied.
Example; α-[1-14C]-Methylaminoisobutyric Acid (catalog number NEC671) - Uniformly Labeled (U) Compounds
This designation is reserved for compounds labeled in all positions in a uniform or nearly uniform pattern. This category includes compounds prepared by biosynthesis from carbon dioxide, [14C], or from a uniformly labeled intermediate.
Example: Isoleucine,L-[14C(U)]- (catalog number NEC278E) - Nominally Labeled (N) Compounds
This designation is used when the method of preparation requires some (usually a significant amount) of the label to be at a specific site or sites, but no further information is available on the extent of labeling (if any) at other positions.
Example: Enkephalin (2-D-Alanine-5-D-Leucine), [Tyrosyl-3,5-3H(N)]- (catalog number NET648) - Generally labeled (G) compounds
This designation is reserved for compounds (usually tritium-labeled) in which there is a random (non-uniform and undetermined) distribution of radioactivity at various positions. Under the conditions that lead to random labeling, many potential sites for labeling usually contain no radioactivity.
Example: Dihydroxyphenylethylamine, [3H(G)]- (Dopamine) (catalog number NET1160
Custom radiosynthesis and radiolabeling
Revvity offers custom services for radiolabeling your protein or nucleic acid of interest, or for synthesizing a radiochemical to meet your specific needs. If you are interested in these services, please contact our custom teams:
Custom Radiolabeling and Radiosynthesis Services
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























