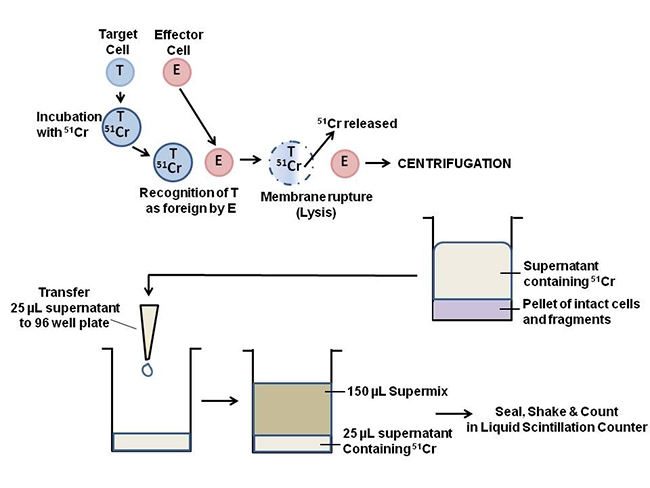
Overview
Chromium-51 (51Cr) release assays are commonly used for the precise and accurate quantification of cytotoxicity, particularly in the study of tumor and viral cytolysis. The assay is used to determine the number of lymphocytes produced in response to infection or drug treatment.
A brief overview of the assay principle is illustrated below. Target cells are labeled with Chromium-51 (51Cr) release assays are commonly used for the precise and accurate quantification of cytotoxicity, particularly in the study of tumor and viral cytolysis. The assay is used to determine the number of lymphocytes produced in response to infection or drug treatment. A brief overview of the assay principle is illustrated below. Target cells are labeled with 51Cr, the label is then released from the target cells by cytolysis. The label can be isolated by centrifuging the samples and collecting the supernatants. Supernatants from centrifugation can either be counted directly in a gamma counter or mixed with scintillation cocktail in a microplate (or dried on a LumaPlate™) and counted in a liquid scintillation counter.

Figure 1. Principle of the chromium release assay. The procedure can be divided into 3 main steps: 51Cr labeling the target cell, release of the 51Cr label by cytolysis, and detection of the released 51Cr label.
Featured Products

Radionuclides provide a highly specific and sensitive labeling option for proteins, cells, and tissues.

Chromium-51 Radionuclide, 1mCi, EDTA Complex in 0.005M EDTA (pH 7.0)
Chromium-51 Radionuclide EDTA Complex in 0.005M EDTA (pH 7.0) Shipped ambient. Cr-EDTA in 0.005M EDTA pH ~7>50Ci(1.85TBq)/g

Chromium-51 Radionuclide, 1mCi (37MBq), Sodium Chromate in Normal Saline (pH 8-10), Steri-packaged
Chromium-51 (51Cr) radionuclide, commonly used as a label for target cells in chromium release assays, cell-mediated cytotoxicity studies, antibody-dependent cytotoxicity (ADCC) studies, and CAR-T cell development.

Chromium-51 Radionuclide, 1mCi (37MBq), Sodium Chromate in Normal Saline (pH 8-10)
Chromium-51 (51Cr) radionuclide, commonly used as a label for target cells in chromium release assays, cell-mediated cytotoxicity studies, antibody-dependent cytotoxicity (ADCC) studies, and CAR-T cell development.
What do I need to run this assay?
Required to run the general assay:
- Chromium-51 (see next section for Revvity product numbers)
- Cell Culture Media
- Buffer, such as PBS (optional)
- Detergent, such as Triton-X or SDS, to obtain maximum release values
- Tubes or microplates (96 well round bottom) for cell incubations, depending on assay format
- Centrifuge for tubes or microplates, depending on assay format
Required for detection of Chromium-51:
Gamma Counting (e.g., WIZARD2® Gamma Counter):
- Polystyrene vials
Liquid Scintillation Counting (e.g. vial-based counter):
- Polyethylene or glass vials
- Scintillation Cocktail -- Ultima Gold™ (6013321)
Liquid Scintillation Counting (e.g. MicroBeta™ plate-based counters):
- For top reading instruments OptiPlate™ (6005299)
- For bottom reading instruments (MicroBeta) -- Flexible plate (1450-401) or IsoPlate (6005040)
- Optional: LumaPlates™ (6006633) can be used in top reading instruments in place of plates mentioned above. Samples are dried in LumaPlates overnight and counted without the addition of scintillation cocktail.
- TopSeal™ A PLUS (6050185)
- Optional: Plate Shaker
Product and catalog numbers
| Compound | Specific Activity | Rad. conc. | Packaging buffer | Storage temp. | Half Life | Fresh Lot Date | Cat. Number |
|---|---|---|---|---|---|---|---|
| Sodium Chromate | 400-1200 Ci/g | 5 mCi/mL | Saline, pH 8-10, sterile | Saline, pH 8-10, sterile | 27.7 days | Every other Friday | NEZ030 |
| Sodium Chromate | 400-1200 Ci/g | 1 mCi/mL | Saline, pH 8-10, sterile | Room Temperature | 27.7 days | Every other Friday | NEZ030S |
Protocol-in-brief
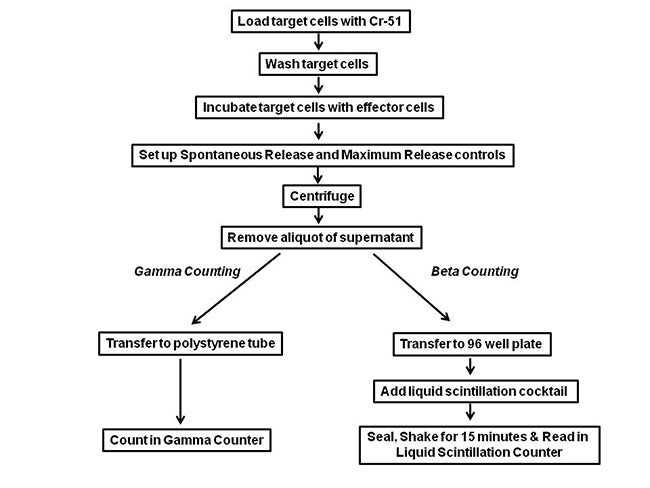
Figure 2. Protocol for chromium release assay. Either Gamma or Beta Counting can be used for Cr51 detection.
Important Controls
Spontaneous Release: Target cells without Effector cells. Incubate Target cells with an equal volume of media or buffer only.
Maximum Release: Incubate Target cells with media or buffer containing 1-2% detergent to completely lyse Target cells (e.g., SDS, Triton X-100).
Percent Specific Lysis: [(Experimental Release – Spontaneous Release)/ (Maximum Release – Spontaneous Release)] 100.
Assay Optimization
Experimental conditions such as the amount of radioactivity used to label target cells, the length of the target cell labeling incubation, E:T ratios, and E: T incubation times will vary based on cell types used and should be optimized for your assay.
Gamma vs. Beta Counting Efficiency
Traditionally, 51Cr is considered a gamma emitter. However, since 51Cr decays by electron capture it can be quantified by detection of the gamma ray in a gamma counter or by the detection of the more abundant Auger electrons and low energy x-rays in a liquid scintillation counting system. This is reflected in the counting efficiency in each method. In a gamma counter, counting efficiency is unlikely to be more than 7%. In an instrument such as the MicroBeta, 51Cr counting efficiency is 26%.
Sample protocol and data
- Infect P815 Target cells (2 x 106) with recombinant vaccinia virus (107 pfu) that expresses the nucleoprotein gene.
- Resuspend Target cells for labeling in 50 µl of IMDM (7.5% FCS) plus 50 µCi of Na251CrO4 for one hour.
- Wash cells and resuspend in IMDM.
- Add 104 Target cells/well of a round-bottom 96 well plate.
- Add Effector cells (CTL taken from BALB/cByJ mice immunized with the recombinant vaccinia virus) at E:T ratios of 27:1, 9:1, 3:1, and 1:1. Incubate 4 hours at 37°C.
- Prepare important controls (Spontaneous Release, Maximum Release) and incubate 4 hours at 37°C.
- Centrifuge samples and collect supernatant.
- Add 30 µl supernatant to a polystyrene tube (for gamma counting) and count on Wizard™.
- OR for liquid scintillation counting, add 30 µl of supernatant to either a PicoPlate™ well plus 250 µl MicroScint™-20 (shake plate to mix), or a LumaPlate™ well and then air dry. Seal plate and count on TopCount.
Sample Data
Obtained from following the above Sample Protocol
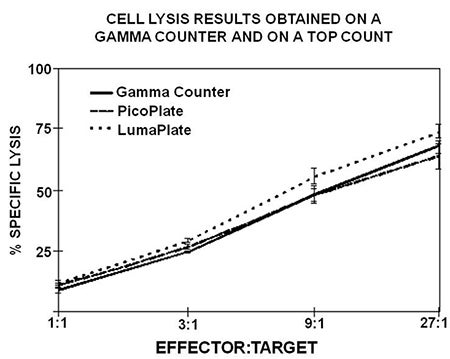
Figure 3. Comparison of the percent specific lysis between supernatants counted in tubes on a gamma counter and counted in LumaPlates or PicoPlates. Killing measured via gamma counting had a range of 9.5% to 68.2%, while Counter produced similar ranges using PicoPlates, 11.7% to 63.9%, and LumaPlates, 12.3% to 73.5%.
Citations
- Mickel, R.A., Kessler, D.J., Taylor, J.M, and Licktenstein, A. Natural Killer Cell Cytotoxicity in the Peripheral Blood, Cervical Lymph Nodes, and Tumor of Head and Neck Cancer Patients. Cancer Res 48, 5017-5022 (1988). Link
- Olson, L.M. & Visek, W.J. Kinetics of Cell-Mediated Cytotoxicity in Mice Fed Diets of Various Fat Contents. J. Nutr 120, 619-624 (1990). Link
- Lavie, G., Meruelo, D., Aroyo, K., and Mandel, M. Inhibition of the CD8+ T cell-mediated cytotoxicity reaction by hypericin: potential for treatment of T cell-mediated diseases. Int. Immunol 12, 479-486 (2000). Link
- Lamikanra, A., Pan, Z., Isaacs, S.N., Wu, T., & Paterson, Y. Regression of Established Human Papillomavirus Type 16 (HPV-16) Immortalized Tumors In Vivo by Vaccinia Viruses Expressing Different Forms of HPV-16 E& Correlates with Enhanced CD8+ T-Cell Responses That Home to the Tumor Site. J. Virol 75, 9654-9664 (2001). Link
- Schmidt, K.N., Leung, B., Kwong, M., Zarember, K.A., Satyal, S., Navas, T.A., Wang, F., and Godowski, P.J. APC-Independent Activation of NK Cells by the Toll-Like Receptor 3 Agonist Double-Stranded RNA. J. Immunol 172, 138-143 (2004). Link
- Wallace, D., Hildesheim, A. & Pinto, L.A. Comparison of Benchtop Microplate Beta Counters with the Traditional Gamma Counting Method for Measurement of Chromium-51 Release in Cytotoxic Assays. Clin Vaccine Immunol 11, 255-260 (2004). Link
Other Revvity assays for cytotoxicity
DELFIA™ TRF cell cytotoxicity assay
Custom conjugation and custom assay development at Revvity
Revvity offers custom radiolabeling and other custom services. If you are interested, please contact us.
Radiosynthesis and Labeling Custom Services
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























