Overview
Radiolabeled nucleotides are commonly used for detection of specific nucleic acid sequences. They are typically incorporated enzymatically into DNA and RNA sequences for detection and analysis. Labeled nucleotides may be incorporated by a variety of methods including in vitro transcription with SP6, T3 or T7 RNA polymerase, 3’ end labeling with terminal deoxynucleotidyl transferase (TdT), T4 DNA polymerase or T7 DNA polymerase, random primed DNA labeling with Klenow fragment, cDNA labeling with AMV or M-MuLV reverse transcriptase, nick translation labeling with DNAse 1 and DNA Polymerase 1, and PCR labeling with thermophilic DNA polymerases like Taq or Pfu. The resulting labeled probes may then be used in applications such as in situ hybridization, microarray analysis, DNA sequencing, southern blotting and northern blotting.
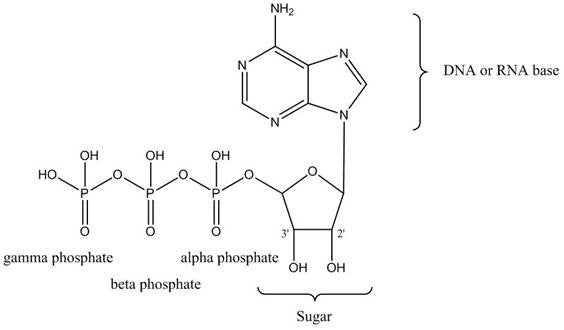
Revvity offers a wide range of 32P and 35S labeled nucleotides, stocked at different concentrations and specific activities to meet your application. Our radionucleotides are labeled on either the alpha phosphate group, or the gamma phosphate group. This designation does not refer to the type of energy that is emitted. 32P and 35S are all beta-emitters (they give off beta energy in the form of beta particles). The "alpha" and "gamma" designation refers to the phosphate group of the nucleoside triphosphate that is labeled with the radioisotope. If you are performing an enzymatic assay (phosphorylation with a kinase, end labeling an oligonucleotide with terminal transferase, etc.), it is important to consider how your enzyme is using the nucleotide as a substrate. You will need to understand how your enzyme works. Is it incorporating the nucleoside monophosphate (base, sugar, and alpha phosphate group) as part of a DNA or RNA chain? Is it transferring the terminal (gamma) phosphate group from the nucleotide to another substrate? Do you need to use a nucleotide that is deoxy at the 2' position of the sugar (a deoxyribonucleotide, dNTP)? Do you need a "chain terminator" that is deoxy at the 3' position?

Figure 1. Structure of ATP. The entire molecule comprises a nucleotide (also called a nucleoside triphosphate). The alpha, beta, and gamma phosphate groups are indicated. This is not a deoxyribonucleotide, because there is a hydroxyl group at the 2' position of the ribose sugar. This would also not be a chain terminator, as there is a potentially reactive hydroxyl group at the 3' position.
For help to select the appropriate radiolabeled nucleotide please see Which formulation should I choose?
DNA and RNA labeling techniques
Oligonucleotides can be labeled at either the 3' or the 5' end. Using polynucleotide kinase and ATP-gamma-32P, the 5' end is labeled. Using terminal transferase and deoxynucleotide triphosphate labeled on the alpha phosphate, the 3' end is labeled. Traditionally, the isotope of choice has been 32P, however 35S has been used successfully. Use of 35S is especially useful when high resolution (as in in situ hybridization) or when long probe stability is needed.
Table 1. Description of some common DNA and RNA labeling methods and terms:
| Application | Description |
|---|---|
| Random Primer | The basic technique for primer extension labelling was first introduced by Feinberg and Vogelstein, and used a mixture of hexanucleotides to prime DNA synthesis randomly on single stranded DNA. DNA synthesis, which requires a primer and a template, can be accomplished by either the holoenzyme DNA polymerase I, or the Klenow fragment of DNA polymerase I. Because Klenow fragment lacks the 5’ → 3’ exonuclease activity, the use of Klenow fragment in primer extension avoids the loss of incorporated label. This allows routine incorporation of radiolabeled nucleotide at greater than 60%, in less than 30-60 minutes, providing DNA probe specific activities of greater than 109 dpm/µg DNA. |
| Nick Translation | In nick translation, the DNA is treated with DNase to produce single-stranded "nicks." DNA polymerase I is then used to replace the nicked sites, elongating the 3' hydroxyl terminus, removing nucleotides by 5'-3' exonuclease activity, and replacing with dNTPs. To radioactively label a DNA fragment for use as a probe, one of the incorporated nucleotides provided in the reaction is radiolabeled on the alpha phosphate position. The translated nick can be sealed by DNA ligase. |
| Klenow | Klenow fragment lacks the 5' → 3' exonuclease activity of DNA polymerase I, which can be advantageous in preparing radiolabeled probes. Klenow fragment can be used to label the termini of DNA fragments by using radiolabeled dNTPs to fill recessed 3' termini, or to end-label DNA molecules with protruding 3' tails. |
| Reverse Transcriptase | In this reaction, RNA is reverse transcribed to cDNA using reverse transcriptase. |
| Thermophilic DNA Polymerase | Like other DNA polymerases, thermophilic DNA polymerases catalyze synthesis of DNA from nucleotide triphosphates. These thermostable enzymes are used frequently in PCR. A free 3' hydroxyl is required to initiate synthesis and magnesium ion is necessary. In general, optimal activity is achieved at 75 degrees C to 80 degrees C. At lower temperatures, the activity is reduced. Some common thermophilic DNA polymerases are Taq, Pfu and Vent. |
| DNA 3' End and DNA 5' End labeling | The techniques for end labeling oligonucleotides with radioisotopes have driven nucleic acid probe technology. Oligonucleotide probes can be custom made based on sequence information of the target DNA or RNA in several hours on a DNA synthesizer. Use of a DNA synthesizer eliminates the usual cumbersome and time-consuming steps involved in cloning and isolation of restriction fragments to be used as probes. Oligonucleotide probes are highly specific and can be designed to detect single base changes in a gene. 3' end labeling of DNA is usually carried out using terminal transferase. 5' end labeling of DNA (or RNA) is usually carried out using T4 PNK. |
| SP6 | SP6 is a DNA-dependent RNA polymerase. It uses a DNA template that has a specific SP6 phage promotor. SP6 is used in the synthesis of labeled RNA probes for use in hybridization. SP6 DNA-dependent RNA polymerase, like T7 RNA polymerase, can be used to synthesize RNA sequences from short DNA templates which contain the appropriate 18 base pair promoter region. Use of SP6 polymerase extends the range of possible 5' sequences of RNA products since the preferred SP6 start site (of the RNA product) is 5'GAAGA, while T7 polymerase prefers 5'GGGAG. The SP6 start site can be advantageous in large-scale syntheses, where high concentrations of RNA can lead to aggregation. SP6 works best with UTP, CTP, and GTP but not well with ATP. |
| T7 RNA polymerases | T7 RNA polymerase catalyzes the formation of RNA from a DNA template containing the appropriate promoter. T7 polymerase is extremely promoter-specific and only transcribes bacteriophage T7 DNA or DNA cloned downstream of a T7 promoter. This reaction requires a DNA template and Mg2+ ion as cofactor for the synthesis of RNA. T7 RNA polymerase activity is stimulated by BSA or spermidine. In contrast to bacterial RNA polymerases, T7 polymerase is not inhibited by the antibiotic rifampicin. T7 works well with UTP, CTP, and ATP but not with GTP. |
| RNA 3' End labeling | T4 RNA ligase can be used to 3'-end label RNA molecules. The enzyme catalyzes the ligation of the 5' phosphate terminus of a radiolabeled nucleotide to the 3'-hydroxyl terminus of a single-stranded DNA or RNA oligo in an ATP-dependent manner. |
Featured Products
Radionucleotides
We offer a wide range of 32P and 35S labeled nucleotides, stocked at different concentrations and specific activities to meet your application. Use to detect specific nucleic acid sequences.

Radiochemicals
Radiometric detection is considered the gold standard for many applications, from drug discovery and development to plant sciences.
Applications
Please note that all these protocols were developed with an enzyme from a specific supplier. You should use the protocol recommended by the supplier of your enzyme to perform your DNA and RNA labeling assays.
Random Primer Extension Labeling
Click here for more information and details on performing a random primer extension labeling
Nick Translation Labeling
Click here for more information and details on performing a Nick Translation labeling assay
3' End Labeling
Click here for more information and details on performing a 3' End labeling assay
5' End Labeling
Click here for more information and details on performing a 5' End labeling assay
Reverse transcriptase reaction
Click here for more information and details on performing a reverse transcription reaction
Protocol-in-brief: Reverse Transcriptase reaction
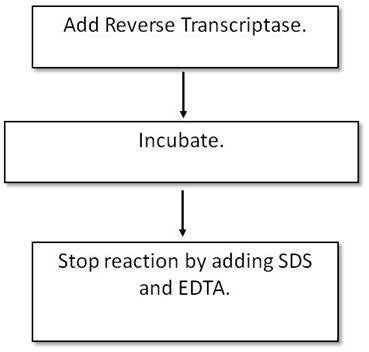
What do I need to run this assay?
Reagents available from Revvity
- Radionucleotides
- Recommended: Charcoal trap (Cat. No. NEX033T) when working with 35S-labeled nucleotides
Reagents needed and available from other suppliers
- Enzyme (appropriate for your assay)
- Any unlabeled ("cold") nucleotides or nucleotide mix necessary
- Reaction buffer
- Template DNA or RNA, or DNA or RNA oligo as appropriate for the reaction
- Incubators, stop solutions as necessary
Citations
- Berent, S. L., M. Mahmoudi, R. M. Torczynski, P. W. Bragg, and A. P. Bollon. 1985. Comparison of oligonucleotide and long DNA fragments as probes in DNA and RNA dot, southern, northern, colony and plaque hybridizations. Bio-Techniques 3: 208-220.
- Friedman, E.Y. & Rosbash, M. The syntheis of high yields of full-length reverse transcripts of globin mRNA. Nucleic Acids Res 4, 3455-3471 (1977). Link
Guides and other resources
- Methods for preparing labeled hybridization probes - tables comparing various labeling techniques, with information on the specific activity that can be achieved by each labeling method, strengths and weaknesses, enzyme used, etc.
Other Revvity Nucleic Acid labeling technologies
Custom conjugation and custom assay development at Revvity
Revvity offers custom radiolabeling. Please contact us if you are interested in having your biomolecule custom labeled.
Radiosynthesis and Labeling Custom Services
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























