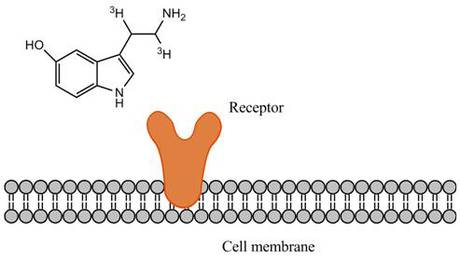
Overview
Radioactive ligands are commonly used to measure ligand binding to receptors. In this assay, you will measure binding of a radiolabeled ligand to cells or cell membranes containing a receptor of interest. Both whole cells and cell membranes can be used for this assay. Radioligands can be used to perform saturation curves, competition, and kinetic experiments.

Ligand-receptor binding
When selecting a radioligand, there are a few factors to keep in mind:
- High specific activity: Radioligands with a high specific activity are well-suited for ligand binding assays. Specific activity indicates how much radioactivity there is per molecule of ligand and is usually given in units of Curies per millimole of ligand. Several of our 125I-labeled ligands are offered at maximum specific activity (2200 Ci/mmol if one 125I labeling site is available, 4400 Ci/mmol if two 125I labeling sites are available, etc.). This indicates that virtually every molecule of ligand provided in the stock vial is radiolabeled. For tritiated ligands (3H ligands), you should ideally pick a ligand that has a specific activity above 20 Ci/mmol. The maximum theoretical specific activity per tritium is 29 Ci/mmol (Curies per millimole of tritium). Specific activities above this value indicate that on average, each molecule of ligand has at least one tritium.
- Low non-specific binding: Hydrophobic ligands will generally show higher non-specific binding. This can sometimes be compensated by coating filters with BSA to reduce non-specific binding. Including BSA, salts or detergents in the wash or binding buffer can also help reduce non-specific binding. If the stock radiochemical is packaged in a silanized vial (refer to the tech data sheet), this may indicate the ligand is somewhat hydrophobic.
- High purity: Ideally, the ligand should have a radiochemical purity above 90%. Radiochemical purity decreases over time, and the actual rate of this degradation accelerates over time. Radiochemical purity and degradation rates for our various radiochemicals can be found on the lot-specific technical data sheets.
- High selectivity: The more selective the ligand is for your receptor, the better your data will be. High selectivity indicates the ligand will mostly be recognized by only one receptor-of-interest, over other receptors that may be present in a cell membrane. Also, using membranes that over-express the receptor of interest may help reduce any potential contribution from receptors endogenously expressed in the membrane.
- Stability: If you will need to use your radioligand over an extended period of time, stability may be a factor for you. 125I-labeled ligands should generally be used within one to two months of the manufacture date. Tritiated ligands should usually be used within 3-6 months of manufacture date; however, there are exceptions to this. Degradation rates and manufacturing dates can be found on our lot-specific technical data sheets. You can also contact technical support to discuss the recommended use time for each Revvity radiochemical - our contact information is on the upper right-hand corner of this page.
- Energy: 3H releases beta energy, which can be measured on a scintillation counter after the addition of scintillant (in the form of a scintillation cocktail, Meltilex™ solid scintillant, an SPA bead, etc.). The beta energy interacts with the scintillant to produce photons, which are measured by the detector. 125I releases both beta-like energy and gamma energy. If you only have access to a gamma counter, you should use a radioligand labeled with 125I. An additional factor is assay format. 125I gives off more beta-like energy than 3H. For SPA assays, either 3H or 125I-labeled ligands can be used effectively.
Radioactive ligand binding assays can be performed in several different formats. Typically, we perform this assay in filtration format, but the assay can also be performed in SPA format. SPA format is a homogeneous format, which means no wash steps are required. In the filtration assay, you will wash away unbound ligand using a vacuum manifold or cell harvester.
| SPA Assay | Filtration Assay | |
|---|---|---|
| Format | Homogeneous | Wash-based |
| Advantages |
|
|
| Disadvantages |
|
|
| Recommended for | Low-throughput (just a few assays) or high throughput; automation; reducing amount of radioactive waste generated | Low-throughput assays or just a few experiments; may be desirable if your lab is already set up for radioactive filtration assays |
Assay Formats
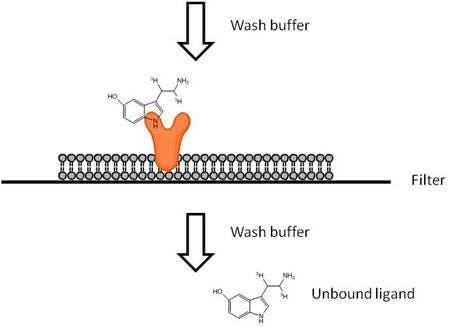
Filtration Ligand Binding Assays
View more information on what materials you may need, what optimizations you may need to perform, and references for radioactive ligand binding filter plate assays. In filtration format, the binding assay is carried out first in one assay plate, then filtered through a filtermat or UniFilter™ plate using a cell harvester (vacuum manifold). The filters are washed to remove any unbound radioligand. The filtermat or UniFilter plate is then dried, and scintillation cocktail (or Meltilex™) is added before reading in an appropriate detector.
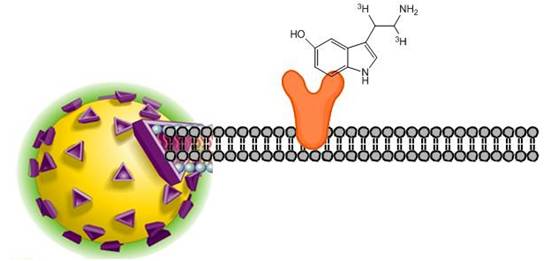
SPA ligand binding assays
View more information on what materials you may need, what optimizations you may need to perform, and references for SPA radioligand binding assays. In the SPA format, cell membranes are captured onto SPA beads. When radioligand binds to the receptor/membrane, this puts the radiochemical into proximity of the SPA bead. The beta energy from the radioligand can interact with scintillant in the bead, producing a signal that can be measured. Radioligand that is not bound to the cell membrane will not be close enough to the SPA bead to interact strongly with the scintillant.
Overview of ligand binding
Definitions
- Affinity (potency): the tightness with which the ligand binds to the receptor. This is usually expressed as an equilibrium constant, Kd. The lower the Kd value, the higher the affinity.
- Specificity: describes how selective a ligand is for one receptor vs. other similar receptors
- Kd: The concentration where 50% of the receptors are occupied by radioligand
Pharmacological Classifications for Ligands
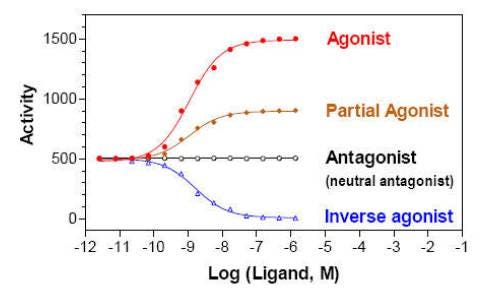
Ligands
- Full agonist: a ligand that binds to and activates a receptor and triggers a maximal response
- Partial agonist: a ligand that binds to a receptor and produces a sub-maximal response
- Antagonist: a ligand that binds to a receptor that does not activate the receptor. Antagonists trigger no response when applied on their own. They will block the effects of a competing agonist ligand.
- Inverse agonist: a ligand that binds to a receptor and decreases response below basal level
Assays
I. Saturation Experiment: Measures binding equilibrium by performing a titration of radioligand, keeping the amount of receptor constant.
You can use saturation curves to determine Bmax (receptor expression level) and Kd (binding affinity of ligand).
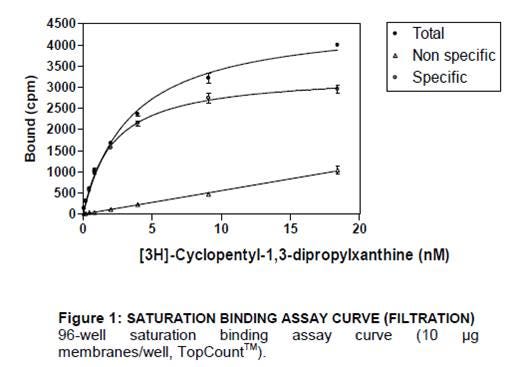
Saturation experiment
- Total binding: Increasing concentration of radioligand in absence of cold ligand. Measures both specific binding to receptor, as well as non-specific binding.
- Non-specific binding: Increasing concentration of radioligand in presence of cold ligand. Measures binding of the radioligand to non-receptor components.
- Specific binding: Total minus non-specific binding. Measures binding to the receptor, specifically.
II. Competition Experiment: Measures equilibrium binding of a single concentration of radioligand in the presence of various concentrations of unlabeled competitor.
You can use competition curves to determine the affinity of many unlabeled compounds for a receptor in screening. IC50 and Ki can be derived from competition data.
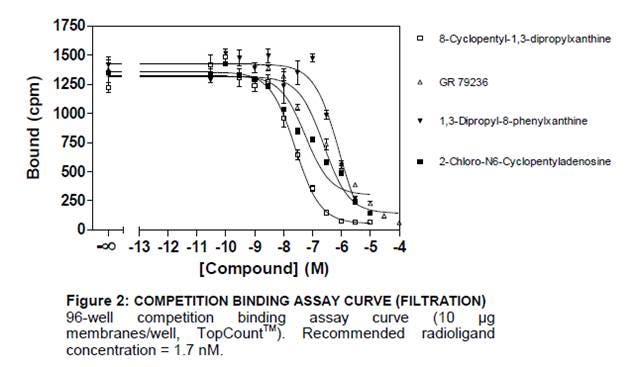
Competition curve
- IC50: Concentration of a competing ligand that displaces half of the radioactive ligand
- Ki: affinity of a cold ligand for the receptor
Note: When changing concentration of radioligand, a shift in IC50 of a reference compound can be observed; however, the Ki stays the same.
III. Kinetic experiments: Measure the level of binding at various times to determine the rate constants for radioligand association and dissociation.
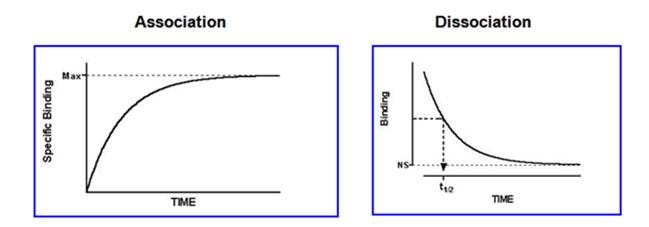
Kinetics
- Saturating concentration of ligand added an amount bound over time to determine the Kon
- High concentration of unlabeled competitor added and decrease in binding over time measured to determine the Koff
Kd = K(off)
K(on)
Tips and FAQs
Ideally, you will want to select a radioligand that has the following properties:
- High specific activity (> 20 Ci/mmol for a tritiated ligand)
- Low non-specific binding (hydrophobic ligands will generally show higher non-specific binding)
- High purity (typically > 90% pure)
- High selectivity
- Stability (if you will need to use your radioligand over an extended period, stability may be a factor. 125I-labeled ligands should generally be used within one to two months of manufacture date. Tritiated ligands should usually be used within 3-6 months of manufacture date (however, there are exceptions to this).
When plotting your data, we recommend that you use the experimentally determined concentration of your radioligand working solutions, rather than a calculated concentration. Some radioligands will stick to the walls of your dilution tubes. It is important to determine the true concentration of your pipetted radioligand. To experimentally determine the concentration of a radioligand dilution, pipette a few microliters out of your dilution tube and spot onto a filtermat. You may also want to spot a 1/10 dilution of this sample, so that you can cross-check your results. Determine the dpms (disintegrations per minute). Convert from dpms to Curies using the following equation:
1 Ci = 2.22 x 1012 dpm
Then use the specific activity of your lot of radioligand to convert from Curies to moles. To determine the radioactive concentration at this point, divide the number of moles by the volume you spotted onto the filtermat.
FAQs
Q. What concentration of radioligand should I use for my saturation curve?
A. We generally recommend you choose 3-5 concentrations below the Kd, and 3-5 concentrations above the Kd. The highest concentration should be ten times the Kd.
Q. What concentration of radioligand should be selected for competitive binding?
A. The radioligand is used at a low concentration, usually at or below its Kd value. If the specific activity is low, concentration above Kd value can be used but it must never be at or higher than saturating concentration.
Q. What type of ligand should I use to determine non-specific binding in my saturation curves?
A. You usually want to choose a ligand that is different than your radioligand. You want to displace only the specific binding of the radioligand to the receptor. You should choose a ligand that has high affinity for the receptor binding site and low affinity for non-specific binding sites.
Other Revvity receptor-ligand binding technologies
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























