Overview
Metabolic labeling of cells with low-energy beta-emitting radioisotopes such as 35S-methionine and35S-methionine/cysteine is often used to follow the biosynthesis, maturation, and degradation of proteins in vivo. Labeling of methionine-containing proteins in cells relies on the use of methionine-free culture media supplemented with a radiolabeled source of 35S. Quantification and identification of the labeled proteins are usually carried out by separation on two-dimensional SDS-PAGE gel and exposing to film (autoradiography) or phosphorimaging (though other methods of detection are possible).
Cell-free protein translation using reticulocyte lysates or wheat germ extracts is also performed using 35S-methionine. Cell-free translation provides a quick way to make small amounts of labeled protein quickly, for use in various types of biochemical assays.
What do I need to run this assay?
Cell labeling assay
- 35S-labeled methionine (refer to table in next section)
- Cells
- Methionine-free culture media
- PBS
- DNase/RNase solution
- Tissue culture equipment
- Gel electrophoresis reagents and equipment
- Film for autoradiography - see Products and Catalog numbers below (unless using a phosphorimager)
- Film developer, or a phosphorimager as appropriate
- Recommended: Charcoal trap (Cat. No. NEX033T)
Cell-free translation
- 35S-labeled methionine (refer to table in next section)
- Cell-free translation system (typically using a reticulocyte lysate or wheat germ extract, such as the TNT® systems from Promega)
- Template RNA (if using an in vitro translation system) or DNA (if using a coupled in vitro transcription/translation system)
- Ribonuclease inhibitor (such as RNasin)
- Recommended: Charcoal trap (Cat. No. NEX033T)
Products and catalog numbers

Radiolabeled 35S Methionine and 35S Methionine/Cysteine products
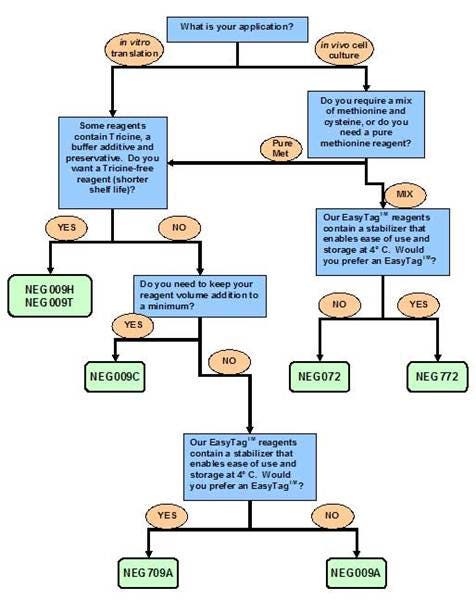
Refer to the flow chart above for guidance in choosing a product for your assay. You can also visit Which formulation should I choose? for additional help to select the appropriate product.
Precautions for handling 35S-labeled compounds
- Solutions containing 35S labeled compounds can release volatile radioactive by-products. In addition to the usual safety practices for handling radioactive material, it is highly recommended to open the vial with a charcoal trap (Cat. No. NEX033T)
- It is advisable when incubating cells with 35S methionine and cysteine that you have charcoal present to capture the volatile by-products. Activated charcoal is available from a variety of vendors.
Autoradiography enhancers for film or phosphorimaging
- EN3HANCE™ Autoradiography Enhancer (Cat. No. 6NE9701): use on gels for best band resolution
- ENLIGHTNING™ Rapid Autoradiography Enhancer (Cat. No. 6NE9741): safer alternative to 6NE9701. Use on gels.
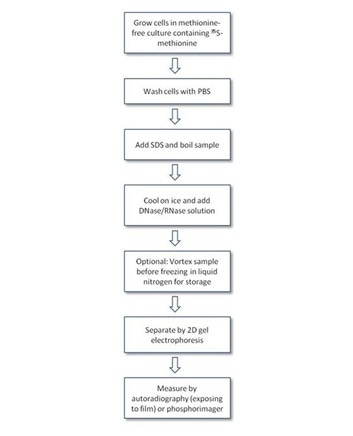
Protocol-in-brief
Cell labeling
Citations
Cell labeling
- Bizios, N. Eukaryotic cell labeling and preparation for 2-D. Methods Mol. Biol112, 49-52 (1999). Link
- Gonnord, P. et al. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J23, 795-805 (2009). Link
- Kolli, B.K., Kostal, J., Zaborina, O., Chakrabarty, A.M. & Chang, K. Leishmania-released nucleoside diphosphate kinase prevents ATP-mediated cytolysis of macrophages. Mol. Biochem. Parasitol158, 163-175 (2008). Link
- Pollard, J.W. The in vivo isotopic labeling of proteins for polyacrylamide gel electrophoresis. Methods Mol. Biol32, 67-72 (1994). Link
- Takahashi, M. & Ono, Y. Pulse-chase analysis of protein kinase C. Methods Mol. Biol233, 163-170 (2003). Link
Cell-free translation
- Kraichely, D.M. & MacDonald, P.N. Confirming yeast two-hybrid protein interactions using in vitro glutathione-S-transferase pulldowns. Methods Mol. Biol177, 135-150 (2001). Link
- Movahedzadeh, F., Rico, S.G. & Cox, R.A. In vitro transcription and translation. Methods Mol. Biol235, 247-255 (2003). Link
- Wilson, C.M. & Bulleid, N.J. Investigation of folding and degradation of mutant proteins synthesized in semipermeabilized cells. Methods Mol. Biol232, 295-312 (2003). Link
Custom services at Revvity
Revvity offers custom radiochemicals, custom plate barcoding and other services. If you are interested in custom services, please contact our custom teams:
Radiosynthesis and Labeling Custom Services
For research use only, not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.






































