

Liquid scintillation counting theory
Liquid scintillation counting (LSC) is the standard laboratory method to quantify the radioactivity of low energy radioisotopes, mostly beta-emitting and alpha-emitting isotopes. The sensitive LSC detection method requires specific cocktails to absorb the energy into detectable light pulses. In order to efficiently transfer the emitted energy into light, LSC cocktails must consist of two basic components:
- The aromatic, organic solvent
- The scintillator(s) or fluors
As the majority of samples applied in LSC are aqueous-based, most of the LSC cocktails consist of:
- The aromatic, organic solvent
- The scintillator(s) or fluors
- The surfactants
Principle of LSC
After excitation of the aromatic solvent molecules through the energy released from a radioactive decay, the energy is next transferred to the scintillator (also sometimes referred to as the "phosphor" or "fluor"). The energy absorbed through the scintillators produces excited states of the electrons, which decay to the ground state and produce a light pulse characteristic for the scintillator. The light is detected by the photomultiplier tube (PMT) of the liquid scintillation counter.
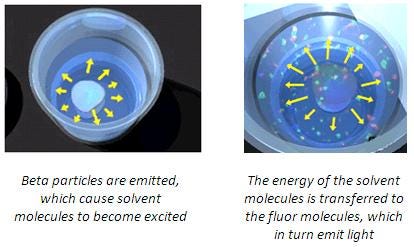
LSC counting principle
Below is a schematic overview of the scintillation process.
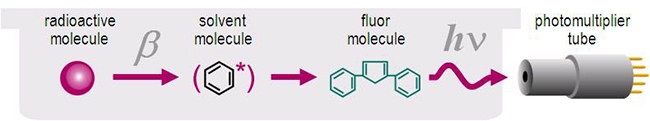
Liquid scintillation counting
Below is a schematic overview of the scintillation process.
Liquid scintillation cocktails
A variety of scintillation cocktails is available to optimize the counting of almost any specific sample. When the sample is purely organic, it can be mixed with a lipophilic cocktail. When the sample is aqueous, or contains even a small proportion of water, then it needs to be mixed with an emulsifying cocktail. Scintillation counting can also be performed using a solid scintillator (for example, MeltiLex™ for Filtermat).
Liquid scintillation cocktails
Solid scintillant alternative (MeltiLex)
Sample preparation
View detailed information on how to prepare various sample types for liquid scintillation counting.
Quench
Read more about quenching and quench correction. Quenching occurs when the energy emitted by a radioisotope is not transferred completely into light and therefore is not detected by the PMT of the counting instrument. The decrease in final signal, as a result of quenching, can occur at various steps of the energy transfer process:
For example:
- The radioisotope can be physically separated from the solution in which the scintillator is dissolved. Properly homogenizing the solution will avoid physical quench.
- The beta particle can be absorbed by so-called quenching agents which will not re-emit the energy, blocking the scintillation process at that stage (the energy of the beta particle will not reach the scintillator, and no light will consequently reach the detector).
- The energy transmission could occur correctly, but once the light is emitted by the scintillator, it may be partially blocked by color quenching. As a consequence, the signal detected at the photomultiplier tube will not represent the total quantity of light truly emitted.
Liquid scintillation vials and plates
Liquid scintillation counting usually requires homogeneous mixing between sample and scintillation cocktail to ensure ultimate contact between analytes and solutes. Good mixing is needed in vials as well as microtiter plates. Many plates and vials exist, and the choice of what type of vial or plate to use will depend on factors such as volume, chemical resistance, safety, and performance in combination with the cocktail of choice. You can view our Vials for Liquid Scintillation Counting and Microplate products to choose a vial or plate adapted to your application.
Glass Vials
Glass provides unparalleled optical clarity (good visibility) and is chemically inert, making it suitable for use with aggressive reagents and solvents. However, glass vials can break when falling on the ground, increasing the risk of contamination.
Borosilicate (Pyrex) glass is preferred due to its lower content of potassium, as compared with soda-glass. Potassium-40 is the largest contributor to background in glass vials. A maximum volume of 20 mL is fixed due to the dimensions of current photomultiplier tubes (2 inch diameter).
| Product Number | Description | Nominal Volume | Caps |
|---|---|---|---|
| 6000167 | Pico Glass Vial | 7.0 mL | Foil-lined urea screw caps |
| 6000096 | Econo Glass Vial | 20.0 mL | Foil-lined urea screw caps |
| 6000128/6000129/6000349 | High Performance Glass Vial | 20.0 mL | Foil-lined urea screw caps |
| 6000134 | High Performance Glass Vial | 20.0 mL | Poly-cone lined urea screw caps |
| 6001009 | Oximate Vial | 20.0 mL | Foil-lined urea screw caps |

Glass vials for scintillation counting
Plastic Vials
Plastic exhibits lower background levels than glass but be aware of static electricity. For this reason, anti-static vials are available. Plastic is combustible and therefore easier for waste disposal. In addition, it is shatterproof. Plastic (polyethylene) is produced from fossil petrochemicals and therefore is preferred because these raw materials contain minimal measurable background.
| Product Number | Description | Nominal Volume | Caps |
|---|---|---|---|
| 1200-421 | Polypropylene vial for MicroBeta | 4 mL | Push on twist off cap |
| 6000252/6000253 | Pico Pro Vial | 4.0 mL | Push on stay on caps (LDPE) |
| 6000292/6000293/6000592 | Pony Vial | 5.5 mL | Push on twist off caps |
| 6000192/6000193 | Pico Prias Vial | 6.0 mL | Poly screw caps |
| 6000186/6000187 | Pico Hang-in Vial | 6.0 mL | Hang-In poly screw caps |
| 6000288/6000289 | Midi-Vial | 8.0 mL | Push on twist off caps (Polypropylene) |
| 6000480/6000488 | Hinge Cap Vial | 8.0 mL | Attached caps |
| 6000201 | Maxi-Vial | 18.0 mL | Poly screw caps |
| 6001095 | Oximate Vial | 20.0 mL | Foil-lined urea screw caps |
| 6001087 | Super Polyethylene Vial with glass vial thread | 20.0 mL | Foil-lined urea screw caps |
| 6000375/6001075/6008117/6008118 | Super Polyethylene Vial with quick closure | 20.0 mL | Poly screw caps |
| 6000477 | Low Diffusion Polyethylene Vial | 20.0 mL | Foil-lined urea screw caps |
Plastic vials for LSC
Microplates
Scintillation results in the emission of light. In order to gain the best sensitivity, it is recommended to count plate-based assays in white microplates.
If your detection instrument reads from the top of the plate, one can use:
- Opaque white plates for standard radiometric assays
- Scintillant-embedded plates2 (LumaPlate™/ScintiPlate)
- Filterplates3 (UniFilter)
If the plates are read from the bottom, such as on a MicroBeta, the plate should have a clear bottom1:
- Clear-bottom plates for standard radiometric assays1 (Isoplate/Flexible plate)
- Scintillant-embedded plates2 (CytoStar-T, ScintiPlate).
1Clear-bottom plates can be turned into opaque plates for top-reading by adding white adhesive BackSeal to the bottom of the plate.
2Scintillant-embedded plates are designed for homogeneous (no-wash) assays. No liquid scintillation cocktail is required, as scintillant is already embedded in the walls of the microplate. In assays using scintillation-embedded plates, separation of "positive" and "negative" signal from the radiochemical is achieved by designing the assay in such a way that the radiochemical is associated with the walls or base of the microplate (and therefore able to interact with the scintillant) under given conditions. For example, in a cell-based uptake assay, radiochemical can only generate signal when taken up by the adherent cells, which are adhered to the base of the plate.
3Filterplates are designed for filtration assays.
Instrumentation
Vial Counters
Tri-Carb™ Series
Tri-Carb Liquid Scintillation Counters are beta counters, and count sample vials with volumes from 4 mL up to 20 mL. They can discriminate between alpha- and beta- radiations, and also allow luminescence measurements.

Quantulus™
The Quantulus Liquid Scintillation Counter is a beta counter dedicated to ultra-low level counting: the thicker shield eliminates effects of cosmic radiations and consequently reduces background. This makes the Quantulus Liquid Scintillation Counter a good choice for Carbon-14-dating.
Plate Counters
In addition to standard protocols using vial counters, radiometric assays can also be performed using higher-throughput detectors such as the MicroBeta2™ microplate counter for radiometric and luminescence detection.

Featured Products
Radiometric Detectors
Radiometric detection has long been and remains today as the gold standard used by researchers in many scientific applications such as analyzing drug pathways.

Scintillation Cocktails
Our liquid scintillation cocktails are convenient, easy-to-use, save preparation time and minimize laboratory errors.

Vials, Caps, & Accessories
For all of your analytical and biological applications, our vials, caps, and septa are made from the highest quality materials.

Microplates
Choosing the right microplate is a critical, often overlooked, part of an assay.

Radiochemicals
Radiometric detection is considered the gold standard for many applications, from drug discovery and development to plant sciences.
References / Useful Links
The liquid scintillation counting technique can be applied to hundreds of radiometric applications, and each will require a scintillation cocktail. Almost all cocktails will give counting results with any application, but the quality and reproducibility of the data will depend on the choice of the cocktail as well as on the sample composition, volume, temperature, and counting device.
Contact our team who can confirm your choice or can recommend the best cocktail for a particular application.
For research use only, not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment




























