All liquid scintillation cocktails (LSC) contain at least an organic solvent and one or more scintillators. The major part of the LSC cocktails, the so-called emulsifying cocktails, contain a combination of surfactants (detergents) to be able to hold aqueous samples.
Role of solvent
When a radioactive decay event occurs in an LSC cocktail, the probability of absorption of the released energy by the solvent molecules of the cocktail is high.
For this reason,
- the solvent must absorb the energy efficiently
- there must be an efficient energy transfer to the scintillators
- the solvent must not quench
- scintillators (fluors) should have a good solubility in the solvent
Good solvents for Liquid Scintillation Counting are alkylated aromatic organic solvents. Nowadays the solvents are divided into “classical” and “safer” solvents, based on the physical properties. The flashpoint is the main criterion for this division.
Classical solvents
Aromatic organic solvents have proven to be the best solvents for liquid scintillation counting due to the high electron density, which provides a target for β-interaction, capturing the energy of the nuclear decay.
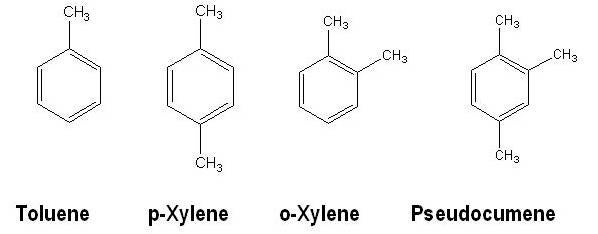
The structures of the four main traditional solvents (Toluene, Xylene (mixture of isomers) and Pseudocumene) are shown below. Other benzene derivatives can be used as well.

These solvents are hazardous by inhalation (and skin absorption) and can be irritating to skin and eyes. They have a high vapor pressure, a low flash point [from 5 - 50°C (flammable)], and storage in laboratories is restricted and should be used in fume hoods. Nowadays in practice Pseudocumene is used exclusively in classical cocktails.
- Insta-Gel™ Plus: as indicated in its name, this cocktail has the property of forming a gel when mixed with large volumes of water. See the acceptance table on the Insta-Gel Plus web page. This property makes it the scintillant of choice for thin layer chromatography (TLC) scrapings and suspended solids.
- Hionic-Fluor™ has been developed to mix aqueous samples with high ionic strength and solubilized samples in strong alkaline media.
- Filter-Count™ has been developed to count radiolabeled samples trapped on filters. Filter-Count has the property to dissolve cellulose nitrate, mixed cellulose esters and PVC filters.
- Pico-Fluor™ Plus are general purpose Pseudocumene-based cocktails exhibiting a high counting efficiency and a large sample acceptance for a wide variety of samples.
- Like all "Flo" cocktails, Flo-Scint™ is a family of flow detection cocktails designed to be used in Flow Scintillation Analyzers (FSA) coupled to HPLC instruments. This family contains various cocktails specifically designed for applications where gradients, especially those with methanol and acetonitrile, need to be counted.
Safer solvents
Why safer, not just “safe”? Although these solvents are much safer in practical use, we must bear in mind that we are dealing with chemicals. All the safety aspects are favorable compared to the classical solvents.
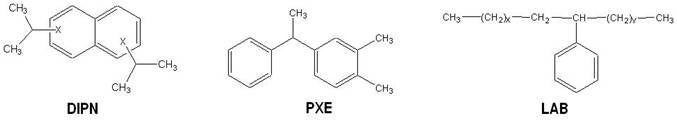
The molecular structures of the three main "safer" solvents used in the composition of scintillation cocktails (DIPN, PXE, LAB) are shown below. These have low toxicity and are classified as harmless for transport & storage. They have a low vapor pressure and a high flash point [above 140°C (non-flammable)].
- The Ultima Gold™ cocktails are a family of high-performance cocktails with very high flash point, low toxicity, high counting efficiency and high quench resistance. This makes these cocktails the family of choice for most common counting applications. Various cocktails can be chosen depending on the type of application: Ultima Gold broadly covers 80% of all counting applications; Ultima Gold AB is specifically designed for alpha-beta discrimination and has an excellent sample holding capacity, making it the ideal for a variety of acidic sample types; Ultima Gold LLT mixes large volumes of water, making it the cocktail of choice for environmental counting of low levels of tritium in water; Ultima Gold F is a lipophilic cocktail providing high counting efficiency with dry filters; Ultima Gold MV is formulated for the rapid uptake of sample in miniature vials and is a good choice for counting wet or damp glass fiber filters; Ultima Gold XR is a high sample load capacity cocktail.
- The Ultima-Flo™ family is the safer alternative to the FloScint cocktails, formulated for acceptance at high mixing ratio gradients of ammonium formate (Ultima-Flo AF), ammonium phosphate (Ultima-Flo AP), methanol and acetonitrile and other HPLC eluents (Ultima-Flo M).
- Opti-Fluor™ cocktails are universal, multipurpose, LAB-based cocktails, with low chemiluminescence, and no permeation through polyethylene vials.
- MicroScint™ cocktails are specifically formulated to be used with samples counted on instruments having TR-LSC PMT (time-resolved liquid scintillation counting photomultiplier tubes), like the MicroBeta2
- The OptiPhase™ family (HiSafe® 2/3, SuperMix, OptiScint HiSafe, BetaPlate Scint) is a DIPN-based cocktail family with high flash point, low toxicity, no permeation through plastic vials and low chemiluminescence.
Ultima Gold has been tested for biodegradability according to Test Method OECD 301E (equivalent to ISO Method 7827 1984). Test requirements were met, showing >70% DOC (dissolved organic carbon) removal within 28 days. A cocktail which passes this test can be described as “Readily Biodegradable”.
Role of scintillator
The molecules of the fluors/scintillator dissolved in the solvent absorb the energy released by the solvent and re-emit this energy (at a higher wavelength) as visible light of a wavelength around 420 nm. The scintillators preferably have a fast decay time and a high Fluorescence Quantum Yield.
The scintillators are divided into two classes, the primary and the secondary scintillators. The emission wavelength of primary scintillators do not fully match the optimum sensitivity of the PMT’s, thus in LSC cocktails a secondary scintillator is present.
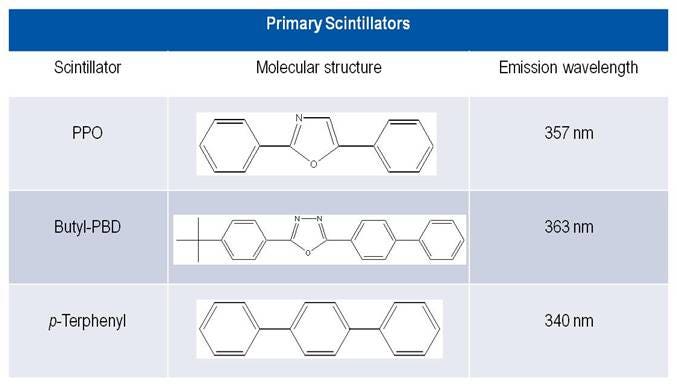
Primary scintillators
Primary scintillators allow direct transfer of energy between excited solvent molecules and the scintillator. The most common primary scintillator is PPO (2,5‐diphenyloxazole). The next common primary scintillator is butyl PBD [2(4-Biphenyl)-5-(4-tert-butylphenyl)-1,3,4-oxadiazole].
Secondary scintillators
The secondary scintillators were used originally as a wavelength shifter to increase the sensitivity of the light for the photomultiplier tube (PMT) to 415-425 nm. The sensitivity of PMTs is lower, below approx. 400 nm. Most primary scintillators emit light below 390 nm, but the response of early photomultiplier tubes drops significantly in this range. A secondary scintillator absorbs the fluorescence energy of the excited primary scintillator and re-emits the energy as a longer wavelength signal.
*While modern phototubes are generally capable of counting the light pulses from the primary scintillator, secondary scintillators have been found to improve efficiency in many cases and are still included in a major part of LSC cocktails.
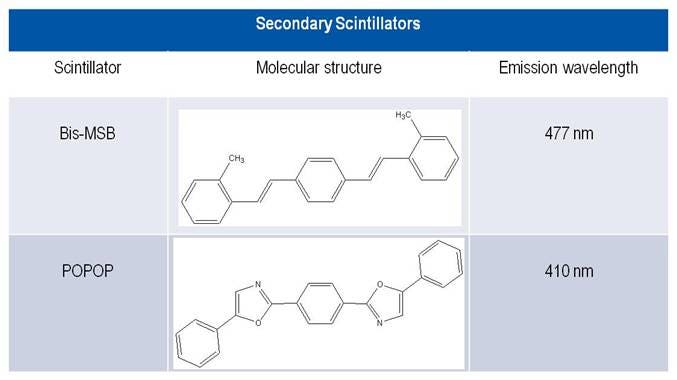
The most common secondary scintillator is Bis-MSB [p-bis-(o-MethylStyryl)-Benzene]. POPOP [1-4,bis-2-(5-Phenyloxazolyl)-benzene] is still apllied in LSC cocktails and plastic scintillators.
Role of surfactant
Most radioactive species are present in an aqueous format, and as such are not miscible with aromatic organic solvents. The presence of emulsifiers (also called detergents or surfactants) in a cocktail enables an aqueous sample to come into intimate contact with the aromatic solvent by forming a stable, clear microemulsion, necessary for stable conditions over the counting period.

Upon addition of water to a LSC cocktail, large aggregates are formed in which the water molecule is in the middle. The aggregate is surrounded by the hydrophilic parts of the surfactant. The non-polar head of the surfactant is directed outside into the direction of the solvent/scintillator molecules; a stable microemulsion is obtained, also called micelle formation.
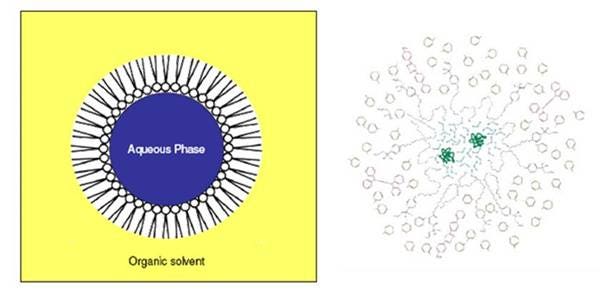
The result is that the water is mixed with an organic solvent with the help of surfactants and the radioactive species present is now still in close contact with the organic solvent.
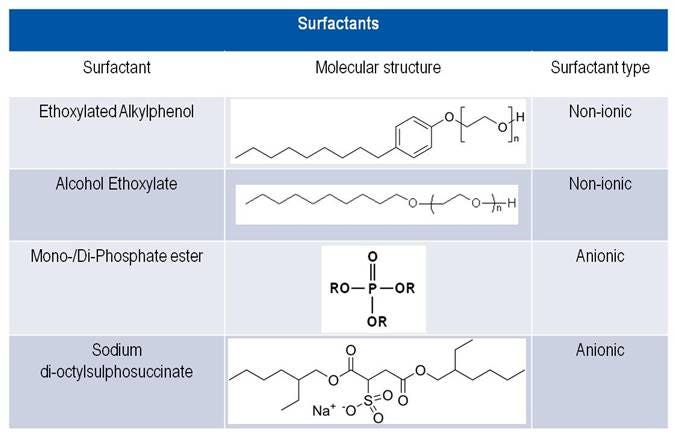
Primarily, two types of surfactants are applied in LSC cocktails:
- Non-ionic: These surfactants have a long hydrocarbon chain on one end and a polar group on the other (e.g., ethoxylated alkylphenols).
- Ionic: These surfactants have a long hydrocarbon chain on one end and a charged group on the other end. They form micelles in the presence of water. This phenomenon is what makes oil and water mix in the presence of surfactant and is called a microemulsion. Ionic surfactants can be of three types: anionic (negatively charged), cationic (positively charged), or zwitterionic (both negatively and positively charged).
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























