Raw data
GTP binding data can be depicted in different ways. One option is to show your raw data, plotting counts per minute (cpm) versus ligand or compound concentration.

Figure 1. Raw data for GTP binding to human adenosine A1 receptor membrane (#ES-010-M). Data was collected in SPA format, using WGA SPA PVT scintillation beads.
Stimulation over basal
Traditionally, GTP binding data is expressed as percentage signal over basal, or fold over basal. This means you are comparing the signal of your unstimulated receptor to the signal of the stimulated receptor. You should subtract the background (non-specific binding) when processing your data.


Figure 2. Equations for determining % signal over basal or fold-over-basal.
If you are calculating % signal over basal or fold-over-basal, you will need to run the following controls to use in the above equation:
- Basal sample: cell membrane, buffer, an 35S-gamma-GTP (no agonist or antagonist)
- Non-specific binding control: cell membrane, buffer, unlabeled GTP-gamma-S, and 35S-gamma-GTP. Excess unlabeled GTP-gamma-S is added prior to the addition of radiolabeled GTP-gamma-S.
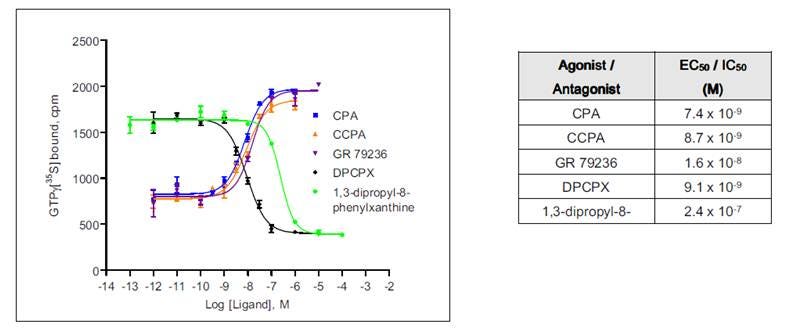
Potency (EC50 or IC50)
Potency is a measure of the concentration of a drug required to generate a particular response. Potency is generally indicated as the EC50 or IC50 of a given ligand or compound for your receptor (the concentration which gives half-maximal response).
Efficacy (Emax)
Efficacy is a measure of the maximum response generated by one ligand in comparison to the maximal response generated by a different ligand. It is a way of normalizing your data. Efficacy is most commonly used to compare the maximal response of agonists to one another.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























