Overview
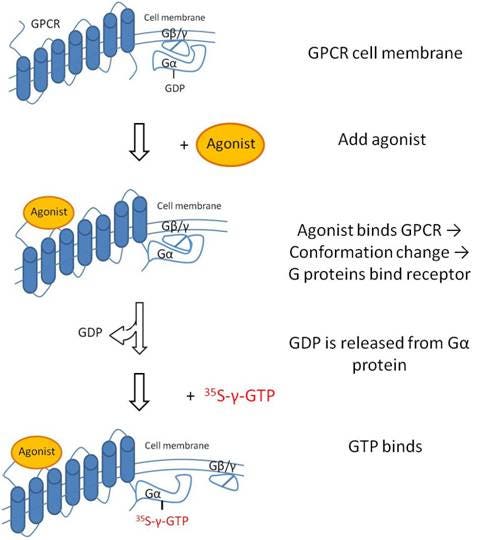
GTP binding is a method used to study activation of GPCRs. In this assay, you will measure binding of a non-hydrolyzable GTP analog to a cell membrane containing an overexpressed GPCR of interest. Cell membranes, rather than whole cells, should be used for this assay. This assay works best with Gi-coupled receptors. Upon activation of the GPCR by an agonist, the receptor changes conformation exposing a binding site for a G-protein complex. Once this G-protein complex is bound, the Gα protein can release GDP and bind GTP. This assay is sometimes referred to as a GTP exchange assay. Because the GTP used in this assay is a radiolabeled non-hydrolyzable analog (35S-gamma-GTP, which is GTP labeled on the gamma phosphate with 35S), you will be able to measure the activation of the GPCR being studied by measuring the amount of radiolabeled GTP bound to the cell membrane. If hydrolyzable GTP was used in the assay, the GTP would be hydrolyzed back to GDP by the GTPase activity of the Gα protein (turnover), removing the radiolabeled gamma phosphate from the radiochemical and leaving no radioisotope associated with the cell membrane for detection.

Figure 1. GTP binding upon activation of a GPCR.
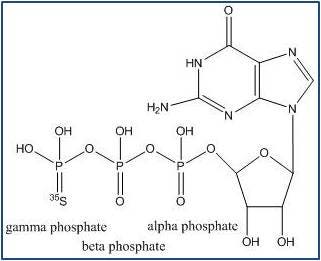
Figure 2. Non-hydrolyzable GTP-gamma-S analog. The molecule is called "GTP-gamma-S" because the gamma phosphate group contains a sulfur atom. The presence of this sulfur in the gamma phosphate group makes the structure non-hydrolyzable at this position. The sulfur also happens to be the labeled atom in this radiochemical. Revvity offers two versions of this product, each in various sizes.
| Product number | Radioactive concentration | Specific activity | Buffer |
| NEG030H | 12.5 mCi/mL | 1250 Ci/mmol | 10 mM Tricine pH 7.6, 10 mM DTT |
| NEG030X | 1 mC/mL | 1250 Ci/mmol | 10 mM Tricine pH 7.6, 10 mM DTT |
GTP binding can be used:
- to determine the potency (EC50) and efficacy (Emax) of the ligand to activate the GPCR-of-interest
- to determine the nature of the ligand: Agonist or Antagonist
- to screen for agonists or antagonists
Radioactive GTPγS assays can be done in several different formats. Typically, we perform this assay in SPA format, but they can also be performed as a filtration assay using filter plates. SPA format is a homogeneous format, which means no wash steps are required. In the filtration assay, you will wash away unbound GTP using a vacuum manifold or cell harvester.
| SPA assay | Filtration assay | |
|---|---|---|
| Format | Homogeneous | Wash-based |
| Advantages |
|
|
| Disadvantages |
|
|
| Recommended for | Low-throughput (just a few assays) or high throughput; automation; reducing amount of radioactive waste generated | Low-throughput assays or just a few experiments; may be desirable if your lab is already set up for radioactive filtration assays |
Assay formats
SPA assays for GTP binding
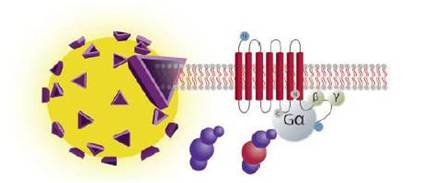
View more information on what materials you need, what optimizations you may need to perform, and references for SPA GTP binding assays. In the SPA format, cell membranes are captured onto SPA beads. When the GPCR is activated, 35S-labeled GTP will bind to the membrane. This puts the radiochemical into proximity of the SPA bead. When the radiochemical is close to the bead, the beta energy from the 35S can interact with scintillant in the bead, producing a signal that can be measured. 35S-GTP that is not bound to the cell membrane will not be close enough to the SPA bead to interact strongly with the scintillant.
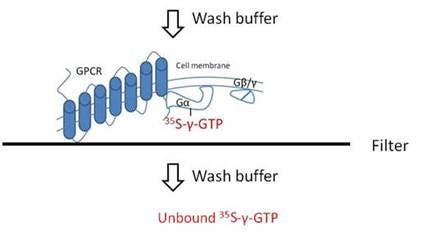
Filtration assays for GTP binding
View more information on what materials you need, what optimizations you may need to perform, and references for radioactive GTP filter plate assays. In the filter plate format, the binding assay is carried out first in one assay plate, then filtered through a filtermat or Unifilter plate using a cell harvester (vacuum manifold). The filters are washed to remove any unbound 35S-gamma-GTP. The filtermat or Unifilter plate is then dried, and scintillation cocktail (or Meltilex) is added before reading in an appropriate detector.
Data analysis
Go to our GTP binding data analysis page.
General resources
- NIH Assay Guidance website for GTP binding assays
- Harrison, C. & Traynor, J.R. The 35S GTP-gamma-S binding assay: approaches and applications in pharmacology. Life Sciences 74, 489-508 (2003). Link
General tips
- The GTPγS assay works best with Gi-coupled GPCRs. Very low assay windows are usually obtained for Gs- and Gq-coupled receptors, due to both (1) levels of expression of Gi relative to Gs or Gq proteins and (2) the exchange rate of these G proteins for GTP.
- The GTPγS assay is sensitive to GDP concentration and concentrations of 35S GTPγS and Mg2+ in the assay buffer.
- If performing a filtration assay, filters for GTPγS assay should not be treated with PEI. This will increase the non-specific binding in the GTP assay.
- The tech data sheets for some of our cell line products show sample data for a GTPγS assay. We have performed the SPA-formatted assay for these membranes. However, this is not a part of the QC performed with each lot of membrane.
- We recommend you perform new assay development and validate assay conditions with well-known ligands. The pharmacological properties of the reference compounds should be identical to those reported in literature.
- Revvity provides custom assay development and assay validation, if you would like for us to optimize the GTP binding assay for you for one of our membrane products.
Custom cell lines, membranes, frozen cells, and receptors
Revvity offers custom cell lines and membranes as well as custom assay development. If you are interested in a custom receptor cell line or membrane, or in custom assay development, please contact our custom teams:
Custom Assay Development Services
For research use only, not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























