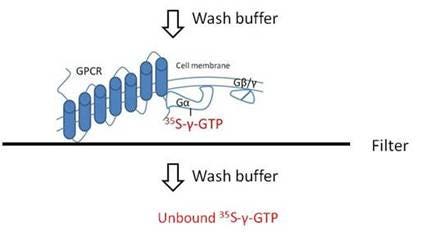
Overview
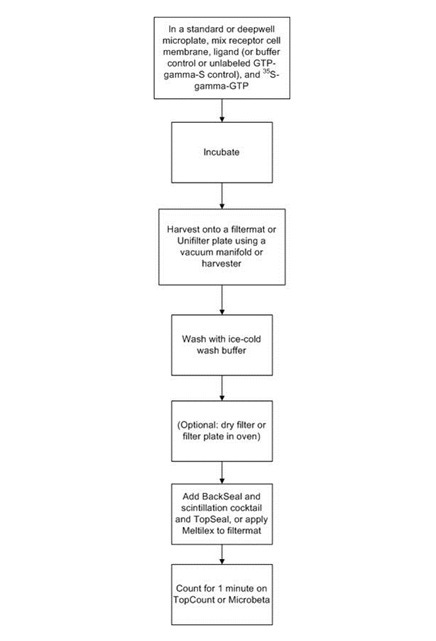
In the filter plate GTP binding format, the binding of 35S-gamma-GTP to the receptor membrane is carried out first in one assay plate, then after incubation the reaction filtered through a filtermat or UniFilter plate. The filters are washed to remove any unbound 35S-gamma-GTP. A cell harvester or vacuum manifold can be used for the filtration and wash steps. The filtermat or UniFilter plate is then dried and scintillation cocktail (or Meltilex™ solid scintillant) is added before reading in an appropriate detector. This filtration format is sometimes favored because you avoid potential issues with proximity effects, which can lead to higher assay background.

What do I need to run this assay?
If you will be using a UniFilter plate with a cell harvester:
- Cell membrane expressing receptor of interest. (Revvity carries receptor-transfected cell membranes)
- 35S-gamma GTP (Cat. No. NEG030H or NEG030X)
- Cold (unlabeled) GTP-gamma-S as a control for non-specific binding
- GDP, agonists, antagonists, test compounds as needed
- Microplate for your binding assay. (We recommend Deep-Well plates, Beckman #267006. These plates have wells with a 1 mL volume capacity. Please check to make sure they will fit in your harvester.)
- Binding buffer (optimized for your assay)
- Wash buffer (optimized for your assay)
- Scintillation cocktail. (We recommend MicroScint-20™ Cat. No. 6013621 if counting slightly damp plates, or Ultima Gold™ MV Cat. No. 6013151 if you are drying your plates prior to reading.)
- BackSeal for plates (Cat. No. 6005199 - this will be used when you are going to add cocktail to the plate.)
- TopSeal™-A for plates (Cat. No. 6050185)
- Cell harvester. (We recommend Filtermate Harvesters - make sure your harvester can accomodate a UniFilter plate)
- High throughput radiometric detector. (We recommend the MicroBeta™ counter)
- Optional: drying oven, set at 27-30 Celsius. Air flow is key, as you don’t want to create vapors while heating.
If you will be using a filtermat and a vacuum manifold:
- Cell membrane expressing receptor of interest. (Revvity carries receptor-transfected cell membranes)
- 35S-gamma GTP (Cat. No. NEG030H or NEG030X)
- Cold (unlabeled) GTP-gamma-S as a control for non-specific binding
- GDP, agonists, antagonists, test compounds as needed
- Microplate for your binding assay. (We recommend Deep-Well plates, Beckman #267006.)
- TopSeal-A™ for plates (Cat. No. 6050185)
- Filtermats, GF/B (Cat. No. 1450-521)
- Omnifilter cassette for fitting filtermat onto assay plate (make sure this is compatible with your vacuum manifold)
- Binding buffer (optimized for your assay)
- Wash buffer (optimized for your assay)
- Filtermat sample bag and scintillation cocktail. (*See note below; we recommend MicroScint-20 Cat. No. 6013621 if counting slightly damp plates, or Ultima Gold™ MV Cat. No. 6013151 if you are drying your plates prior to reading.)
- Optional: drying oven, set at 27-30 Celsius. Air flow is key, as you don’t want to create vapors while heating.
- Vacuum manifold or cell harvester. (We recommend Filtermate Harvesters.)
- High throughput radiometric detector. (We recommend the MicroBeta counter.)
- Cassette for fitting filtermat into detector. (This will depend on which instrument you are using - please contact technical support for more information.)
*You can substitute Meltilex (a solid scintillant that will melt onto the filtermat) and hot plate for melting Meltilex onto the filtermat instead of using a sample bag and cocktail, if you prefer.
35S gamma GTP radiochemicals
Revvity/New England Nuclear offers two different 35S gamma GTP products in various sizes:
| Product number | Radioactive Concentraion | Specific activity | Buffer |
|---|---|---|---|
| NEG030H | 12.5 mCi/mL | 1250 Ci/mmol | 10 mM Tricine pH 7.6, 10 mM DTT |
| NEG030X | 1 mC/mL | 1250 Ci/mmol | 10 mM Tricine pH 7.6, 10 mM DTT |
Protocol-in-brief
Assay optimizations
- Amount of membrane per well (typically 5 - 50 μg/well in 96-well format)
- Binding buffer optimization, including GDP, Mg2+, NaCl, and saponin concentrations. We recommend testing 0-10 μM GDP, 1-10 mM MgCl2, 0-100 mM NaCl, and 3-100 μg/mL saponin.
- Incubation Time (typically 30 min - 1 hour)
- Wash buffer optimization/number of wash steps
- Signal window and Z’ factor (if applicable)
- Evaluation of standard compound concentration response curves
Application note, posters, guides and other resources
- NIH Assay Guidance website for GTP binding assays
Tips and FAQs
- The GTPγS assay works best with Gi-coupled GPCRs. Very low assay windows are usually obtained for Gs- and Gq-coupled receptors, due to both (1) levels of expression of Gi relative to Gs or Gq proteins and (2) the exchange rate of these G proteins for GTP.
- The GTPγS assay is sensitive to GDP concentration, concentration of 35S GTPγS, and concentration of Mg2+ in the assay buffer.
- Filters for GTPγS assay should not be treated with PEI (polyethyleneimine). This increases the non-specific binding.
- Controls: we recommend that when you develop your assay, you include a no-agonist control (substituting with buffer) to determine your basal GTP binding level, and a non-specific binding (NSB) control where you use unlabeled non-hydrolyzable GTP with 35S-gamma-GTP and cell membrane
Data analysis
Visit our GTP binding data analysis page.
Citations
- Chaki, S. et al. Anxiolytic- and Antidepressant-Like Profile of ATC0065 and ATC0175: Nonpeptidic and Orally Active Melanin-Concentrating Hormone Receptor 1 Antagonists. J Pharmacol Exp Ther 313, 831-839 (2005). Link
- Clark, M.J., Furman, C.A., Gilson, T.D. & Traynor, J.R. Comparison of the Relative Efficacy and Potency of μ Opioid Agonists to Activate Gα i/o Proteins Containing a Pertussis Toxin-Insensitive Mutation. J Pharmacol Exp Ther 317, 858-864 (2006). Link
- Heise, C.E. et al. Pharmacological Characterization of CXC Chemokine Receptor 3 Ligands and a Small Molecule Antagonist. J Pharmacol Exp Ther 313, 1263-1271 (2005). Link
- Li, J., Becker, G.L., Traynor, J.R., Gong, Z. & France, C.P. Thienorphine: Receptor Binding and Behavioral Effects in Rhesus Monkeys. J Pharmacol Exp Ther 321, 227-236 (2007). Link
- Lorrain, D.S. et al. Group II mGlu Receptor Activation Suppresses Norepinephrine Release in the Ventral Hippocampus and Locomotor Responses to Acute Ketamine Challenge. Neuropsychopharmacology 28, 1622-1632 (2003). Link
- Okamoto, T. et al. Regulation of Fungal Infection by a Combination of Amphotericin B and Peptide 2, a Lactoferrin Peptide That Activates Neutrophils. Clin Diagn Lab Immunol. 11, 1111--1119 (2004). Link
Custom cell lines, membranes, frozen cells, and receptors
Revvity offers custom cell lines and membranes as well as custom assay development. If you are interested in our custom services, please contact us.
Radiosynthesis and Labeling Custom Services
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.






































