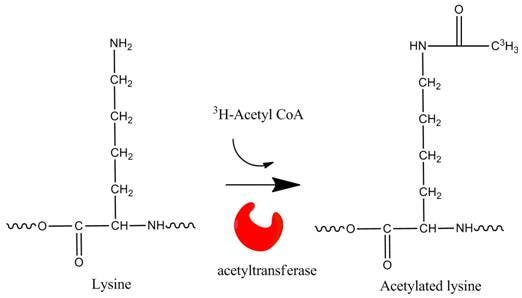
Overview
3H-acetyl CoA and 14C-acetyl CoA are commonly used radiolabeled substrates for studying in vitro acetylation of histones and other proteins. Acetylation typically occurs at either the N-terminus of a protein or peptide, or on lysine residues within a protein. Alternatively, acetylation can be studied in cells using 3H-sodium acetate. Radioactive substrates for deacetylation assays can be prepared using radiolabeled acetate.
N-terminal acetylation is catalyzed by N-alpha-acetyltransferases (NATs). NATs have been identified as drug metabolizing enyzmes, and have been implicated as biomarkers for certain cancers. The physiological role of N-terminal acetylation is an area of active research.
Histone acetylation and deacetylation on lysine residues plays an important role in epigenetic regulation. Acetylation imparts a negative charge that can neutralize the positive charge on histones, reducing the binding of negatively charged DNA. This relaxes chromatin structure, making genes accessible to transcription machinery. The acetylation state of histones is balanced by two classes of enzymes: histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs and HDACs can also acetylate and deacetylate non-histone proteins. HAT and HDAC inhibitors have been studied as potential drugs for treating various cancers.

Acetylation using labeling acetyl-CoA
What do I need to run this assay?
Standard in vitro acetylation assays
- 3H-acetyl CoA or 14C-acetyl CoA (refer to table in next section)
- Protein substrate
- Acetyltransferase enzyme
- Cold acetyl CoA1
- Gels, buffers, and equipment for polyacrylamide gel electrophoresis (PAGE) analysis2
- Autoradiography film and developer2
- ENLIGHTNING™ autoradiography enhancer (Cat. No. 6NE9741)
1Unlabeled acetyl CoA can be used to decrease the specific activity of the stock radiochemical and increase the molar concentration of acetyl CoA in the reaction.
2As an alternative to gel electrophoresis, the reaction can be separated by washing over Whatman P81 paper and measured on a liquid scintillation counter after the addition of liquid scintillation cocktail.
Cell-based acetylation assays
- 3H- or 14C-sodium acetate (refer to table in next section)
- Cells
- Culture media
- Wash buffer
- Purification, lysis or extraction reagents
- Liquid scintillation counter such as a Tri-Carb™ counter, vials and cocktail, or SDS-PAGE reagents with film and developer for autoradiography, or SDS-PAGE reagents with ENLIGHTNING™ autoradiography enhancer and PhosphorImager
Products and catalog numbers
Radiochemicals
| Chemical | Radioisotope | Labeling | Specific activity | Rad. conc. | Solvent | Storage | Cat. number |
|---|---|---|---|---|---|---|---|
| Acetyl-CoA | 3H | acetyl-1 | 1-10 Ci/mmol | 0.1 mCi/mL | Packaged under nitrogen in 0.01 M sodium acetate pH 5 | -20°C | NET290 |
| 14C | acetyl-1 | 40-60 mCi/mmol | 0.2 mCi/mL | Packaged under nitrogen in 0.01 M sodium acetate pH 5 | -20°C | NEC313 | |
| Sodium acetate (acetic acid, sodium salt) | 14C | (position 1) | 50-62 mCi/mmol | 1 mCi/mL | Steri-packaged, aqueous solution | 2-8°C | NEC084A |
| (position 1) | 45-60 mCi/mmol | 1 mCi/mL | Ethanol | 2-8°C | NEC084H | ||
| (positions 1,2) | 45-60 mCi/mmol | 0.1 mCi/mL | Ethanol | -20°C | NEC553 |
How to select a radiochemical from the above table:
- Chemical
- Radiolabeled acetyl-CoA is used for biochemical assays (enzymatic assays)
- Radiolabeled sodium acetate is typically used for cell-based assays
- Radioisotope: Either 14C- or 3H-radiochemicals can be used in acetylation assays. 14C has higher energy compared to 3H. 14C also has higher efficiency compared to 3H in liquid scintillation counting. Your radioactive license may restrict you to one radioisotope or the other. You will want to consult your radiation safety officer when selecting a radioisotope for your assay.
- Packaging
- Radiochemicals that are packaged in ethanol are typically more-stable
- For cell labeling or in vivo experiments, you may need to evaporate off any ethanol in the stock material prior to use, as ethanol can be toxic to cells.
- Specific activity: the higher the specific activity, the greater the amount of radioactivity there is per acetyl CoA or acetate molecule. Specific activity is given in units of Curies per millimole of acetyl CoA or sodium acetate, or milliCuries per millimole of acetyl CoA or sodium acetate. The theoretical maximum specific activity of tritium is ~29 Ci/mmol (Curies per millimole of tritium). 3H products with a specific activity that approaches this value have a greater proportion of molecules labeled with 3H in the stock vial. Note that NET003H has a higher specific activity than NET003 (Ci/mmol vs. mCi/mmol). The theoretical maximum specific activity of 14C is ~62 mCi/mmol (milliCuries per millimole of carbon). 14C products with a specific activity that approaches this value have a greater proportion of molecules labeled with 14C in the stock vial. Specific activity can be decreased by adding cold (unlabeled) acetyl CoA or sodium acetate.
Autoradiography enhancers for film or phosphorimaging
- EN3HANCE™ Autoradiography Enhancer (Cat. No. 6NE9701): use on gels for best band resolution
- ENLIGHTNING™ Rapid Autoradiography Enhancer (Cat. No. 6NE9741): safer alternative to 6NE9701. Use on gels.
Scintillation cocktails
- Ultima Gold™ F (Cat. No. 6013179): can be used as scintillation cocktail for dry filters including glass fiber, cellulose nitrate, cellulose acetate, mixed cellulose esters, and normal paper filters
- Ultima Gold (Cat. No. 6013326): multipurpose liquid scintillation cocktail for aqueous and non-aqueous samples
Scintillation vials
Detection instruments
- Tri-Carb™ liquid scintillation counter
- Quantulus GCT Liquid Scintillation Counter
- MicroBeta™ high-throughput detector for plates (and for some models, 4 mL vials and Eppendorf tubes)
Protocol-in-brief
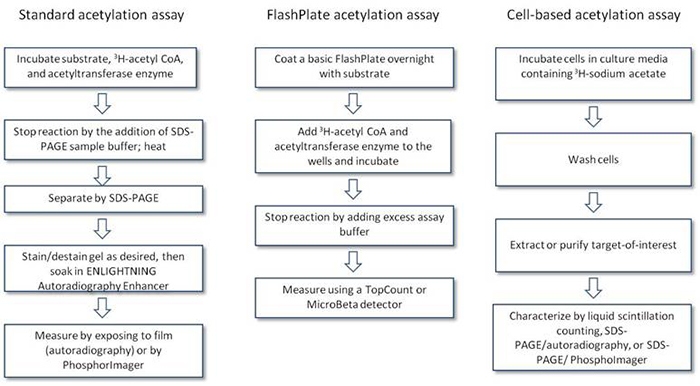
Acetylation workflow
Citations
Standard in vitro acetylation assays
- Pradeepa, M.M. et al. Acetylation of Transition Protein 2 (TP2) by KAT3B (p300) Alters Its DNA Condensation Property and Interaction with Putative Histone Chaperone NPM3. Journal of Biological Chemistry 284: 29956 -29967 (2009). Link
- Stimson, L. et al. Isothiazolones as inhibitors of PCAF and p300 histone acetyltransferase activity. Mol. Cancer Ther 4, 1521-1532 (2005). Link
- Lau, O.D. et al. p300/CBP-associated Factor Histone Acetyltransferase Processing of a Peptide Substrate: KINETIC ANALYSIS OF THE CATALYTIC MECHANISM. Journal of Biological Chemistry 275, 21953-21959 (2000). Link
- Herrera, J.E., Bergel, M., Yang, X., Nakatani, Y. & Bustin, M. The Histone Acetyltransferase Activity of Human GCN5 and PCAF Is Stabilized by Coenzymes. Journal of Biological Chemistry 272, 27253-27258 (1997). Link
FlashPlate in vitro acetylation assays
- Stimson, L. et al. Isothiazolones as inhibitors of PCAF and p300 histone acetyltransferase activity. Mol. Cancer Ther 4, 1521-1532 (2005). Link
- Wynne Aherne, G., Rowlands, M.G., Stimson, L. & Workman, P. Assays for the identification and evaluation of histone acetyltransferase inhibitors. Methods 26, 245-253 (2002). Link
- Turlais, F. et al. High-throughput screening for identification of small molecule inhibitors of histone acetyltransferases using scintillating microplates (FlashPlate). Anal. Biochem 298, 62-68 (2001). Link
Cell-based acetylation assays
- Guo, C., Mi, J., Brautigan, D.L. & Larner, J.M. ATM regulates ionizing radiation-induced disruption of HDAC1:PP1:Rb complexes. Cell. Signal 19, 504-510 (2007). Link
- Kraker, A.J. et al. Modulation of Histone Acetylation by [4-(Acetylamino)-N-(2-Amino-phenyl) Benzamide] in HCT-8 Colon Carcinoma. Molecular Cancer Therapeutics 2, 401-408 (2003). Link
- Verdin, E. et al. Measurement of Mammalian Histone Deacetylase Activity. Chromatin and Chromatin Remodeling Enzymes, Part C Volume 377, 180-196 (2003). Link
Tips
- Published literature suggests that histones can intrinsically attach to the surface of FlashPlates. Therefore, if you are running a histone acetylation assay on FlashPlates, you have the option of mixing the substrate, 3H-acetyl CoA, and enzyme all at once, incubating, then measuring. No pre-coating of the FlashPlate is required.
- Acetyl CoA can be unstable. Very specific conditions are required to prevent hydrolysis. If you are preparing unlabeled ("cold") Acetyl-CoA for your assay, it is usually best to prepare this fresh from powder.
Other Revvity protein acetylation assay technologies
Non-radioactive AlphaScreen® or AlphaLISA™ no-wash acetylation assays
LANCE® TR-FRET acetylation assays
DELFIA® fluorescent histone acetylation and other post-translational modification citations
Custom services at Revvity
Revvity offers custom radiochemicals and custom assay development services. If you are interested in custom services, please contact us:
Radiosynthesis and Labeling Custom Services
For research use only, not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























