Overview
Radioimmunoassays (RIAs) use antibodies to detect and quantitate the amount of antigen (analyte) in a sample. These assays are typically very sensitive and specific. It is possible to detect as low as a few picograms of analyte in the experimental tube when using antibodies of high affinity (Kd = 10-8 - 10-11 M). The basic principle of radioimmunoassay is competitive binding, where a radioactive antigen ("tracer") competes with a non-radioactive antigen for a fixed number of antibody or receptor binding sites. When unlabeled antigen from standards or samples and a fixed amount of tracer (labeled antigen) are allowed to react with a constant and limiting amount of antibody, decreasing amounts of tracer are bound to the antibody as the amount of unlabeled antigen is increased.
In our 125I and 3H radioimmunoassay kits, separation of the antibody-antigen complexes from free antigen is achieved by precipitation of the antibody-bound tracer with either a secondary antibody solution directed against the genus or species specific immunoglobulins of the primary antibody, or by use of polyethylene glycol. Both precipitators generally require the presence of carrier immunoglobulin. After centrifugation, the supernatant containing the unbound antigen is decanted, and the pellet containing the antibody-antigen complex is counted in a scintillation counter. Results obtained for the standards are used to construct a standard (dose-response) curve from which the unknowns are calculated by interpolation.

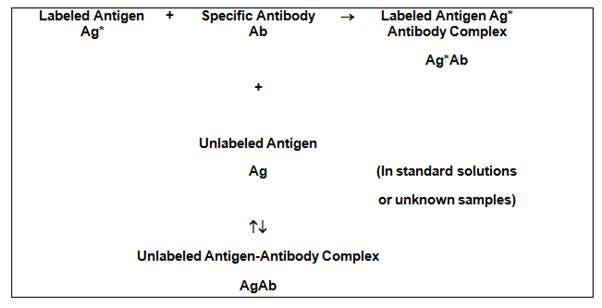
Figure 1. Principle of a competitive binding radioimmunoassay. Radiolabeled antigen ("tracer") added to an antibody specific to the antigen leads to formation of an antigen-antibody complex. Unlabeled antigen from a sample or standard solution can also bind antibody, leading to unlabeled antigen-antibody complex. In the radioimmunoassay, the amount of radiolabeled antigen (tracer) is held constant. Increasing amounts of unlabeled antigen in the sample will compete with tracer for binding to the antibody, leading to more unlabeled antigen-antibody complex.
Figure 2 illustrates how a limited amount of antibody binding sites contained in a test tube or microplate well can either bind unlabeled ligand (in this example, Hepatitis B Surface Antigen) or radiolabeled ligand. As the amount of unlabeled ligand increases, there is consequently less radiolabeled ligand bound. The unlabeled ligand can come from either a “calibration standard” or the sample that you are trying to measure.
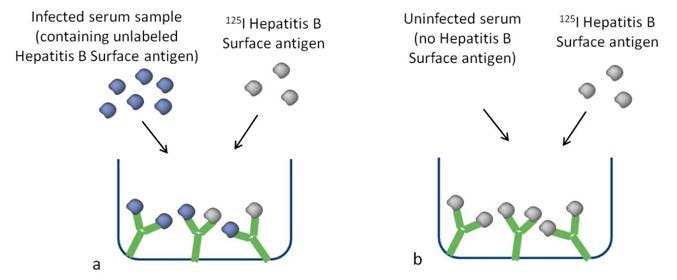
Figure 2. a) Sample containing a high amount of antigen. The unlabeled antigen competes for binding to the antibody in the tube or well. b) Sample containing no or low amounts of antigen. Antibody is bound by the radiolabeled antigen (tracer).
What do I need to run this assay?
Assay Buffer
-
A typical buffer might be 50 mM phosphate pH 7 with 0.9% NaCl and 0.05% sodium azide. To minimize nonspecific antigen “sticking” to the reaction tubes, 0.3% bovine serum albumin may be added. Other additives may include 10 mM EDTA and/or 0.5% Tween-20.
Radiolabeled 125I- or 3H- tracer. Two terms must be understood when optimizing an RIA tracer concentration:
- Specific Activity: This is the amount of radioactivity that is present on the labeled antigen (ligand). The units are usually Ci/mmol or µCi/µg. By knowing this value, the mass of diluted tracer per unit volume in the assay is known. The ideal mass concentration of tracer in an assay is set at slightly below the saturating concentration for the antiserum dilution used in the assay. Ideally, 30-60% of the labeled ligand (zero standard binding) should be bound in the absence of unlabeled ligand at the appropriate tracer mass and antiserum dilution. This provides optimum assay sensitivity. Radioligands with a high specific activity are well-suited for ligand binding assays. Specific activity indicates how much radioactivity there is per molecule of ligand and is usually given in units of Curies per millimole of ligand. Several of our 125I-labeled ligands are offered at maximum specific activity (2200 Ci/mmol if one 125I labeling site is available, 4400 Ci/mmol if two 125I labeling sites are available, etc.). This indicates that virtually every molecule of ligand provided in the stock vial is radiolabeled. For tritiated ligands (3H ligands), you should ideally pick a ligand that has a specific activity above 20 Ci/mmol. The maximum theoretical specific activity per tritium is 29 Ci/mmol (Curies per millimole of tritium). Specific activities above this value indicate that on average, each molecule of ligand has at least one tritium.
-
Activity Concentration: This is the concentration, usually in µCi/mL, of radioactivity per unit volume of solvent or diluent. It has little to do with the actual optimization of tracer mass but serves as a required measurement of the correct amount of counts added to the assay. This is important for the user to know because the calculation of non-specific binding (NSB), zero standard binding and all other binding measurements depend on this.
*In most RIA kits the tracer concentration has already been optimized, and all that is necessary is to perform the recommended dilution in assay buffer and use the appropriate volume per assay tube.
**Pipets and/or pipet tips used to transfer the tracer solution must be of polypropylene or siliconized glass. Do not use those made of unsiliconized glass, as there may be sticking issues.
There are a few additional factors to keep in mind when selecting a tracer for your assay:
- Non-specific binding: Hydrophobic tracers will generally show higher non-specific binding. By including BSA, certain salts or detergents in the assay buffer you can help to reduce non-specific binding. If the stock radiochemical is packaged in a silanized vial (refer to the tech data sheet), this may indicate the ligand is somewhat hydrophobic.
- High purity: Ideally, the tracer should have a radiochemical purity above 90%. Radiochemical purity decreases over time, and the actual rate of this degradation accelerates over time. Radiochemical purity and degradation rates for our radiochemicals can be found on each lot-specific technical data sheet.
- High selectivity: The more selective the tracer is for your antibody or binder, the better your data will be. High selectivity indicates the tracer will mostly be recognized by appropriate antibody binding sites.
- Stability: If you need to use your radiolabeled tracer over an extended period of time, stability may be a factor for you. 125I-labeled ligands should generally be used within one to two months of the manufacture date. Tritiated ligands should usually be used within 3-6 months of manufacture date; however, there are exceptions to this. Degradation rates and manufacturing dates can be found on our lot-specific technical data sheets. You can also contact technical support to discuss the recommended use time for each Revvity radiochemical.
- Energy: 3H releases beta energy, which can be measured on a scintillation counter after the addition of scintillant, in the form of a scintillation cocktail. The beta energy interacts with the scintillant to produce photons, which are measured by the detector.
- 125I releases both beta-like energy and gamma energy. If you only have access to a gamma counter, you should use a radioligand labeled with 125I.
Antiserum
-
For optimized RIA kits, the antiserum (binder) is provided at a concentration chosen to give optimized assay calibration curve sensitivity. It should be at an appropriate dilution to provide 30 to 60% binding of the tracer’s total counts when added in the absence of any unlabeled ligand. If the zero standard (no analyte standard) binding is outside this range, the assay results may not be valid (see Tips and FAQs section, further below).
The antiserum’s avidity and affinity constant properties are the primary determinants of the appropriate concentration range for the calibration curve and the concentrations of analyte that can be measured in the assay. These are properties of the raw antiserum and important factors in the selection screening of appropriate antisera in kit assay development.
In optimizing your own RIA, assay sensitivity is optimized by using an appropriately titered dilution of antisera. This usually gives 30-60% zero standard (no analyte) binding and produces the optimum EC50 calibration curve mid-point. This is always pre-determined in a commercially manufactured kit.
Standard Concentrate
- In most RIA kits, a solution of unlabeled calibration Standard Concentrate is provided along with a dilution protocol to prepare a series of concentrations for a calibration curve. A hypothetical RIA kit may contain a 100 ng/mL Standard Concentrate, which is diluted as follows to prepare a calibration curve appropriate for the assay:
Table 1. Serial dilution of calibration standard.
| Suggested Dilution Scheme for 100 ng/mL stock Calibration Standard | ||
|---|---|---|
| Tube | Concentration (pg/0.1 mL)* | |
| a | 0.1 mL (100 µL) standard + 1.9 mL assay buffer | 500 |
| b | 0.4 mL of dilution a + 0.6 mL assay buffer | 200 |
| c | 0.4 mL of dilution b + 0.4 mL assay buffer | 100 |
| d | 0.4 mL of dilution c + 0.4 mL assay buffer | 50 |
| e | 0.4 mL of dilution d + 0.4 mL assay buffer | 25 |
| f | 0.4 mL of dilution e + 0.6 mL assay buffer | 10 |
| g | 0.4 mL of dilution f + 0.4 mL assay buffer | 5 |
| h | 0.4 mL of dilution g + 0.6 mL assay buffer | 2 |
*This concentration represents actual mass added to assay tube. Dilutions b through h should be used for the standard curve.
**Pipets and/or pipet tips used to transfer diluted standard must be made of polypropylene or siliconized glass.
***Calibration Standards must be diluted fresh on the day of the assay.
Precipitating Reagent
-
The Precipitating Reagent allows the separation of bound ligand-antibody complexes from free ligand and antibody remaining unbound. A solution containing 16% polyethylene glycol (PEG 6000) and 0.05% sodium azide in 50 mM phosphate buffer, pH 6.8 may be used. Alternatively, an anti-isotype specific secondary antibody (such as sheep anti-rabbit serum to precipitate rabbit anti-ligand) may be used. Some carrier rabbit serum is generally required.
Other precipitating reagents include secondary antibody-coated Scintillation Proximity Assay (SPA) beads or coated magnetic particles.

Figure 3. Schematic for a radioimmunoassay. Radioactive antigen ("tracer") is added to the antibody, followed by addition of unlabeled antigen (from sample or from standard). The antigen-antibody complexes formed are precipitated using a precipitating reagent (in the example shown, a secondary antibody) to separate bound and free tracer.
Equipment required:
- Pipettors and/or pipets that accurately and precisely deliver the required volumes
- Polypropylene test tubes
- Test tube rack
- Beakers or flasks
- Vortex mixer
- Centrifuge (refrigerated, with swinging bucket rotor)
- Liquid scintillation counter (such as a Tri-Carb™ or MicroBeta™ liquid scintillation counter) or gamma counter (such as a Wizard2 gamma counter) as applicable
Definitions
Affinity (potency): the tightness with which the ligand binds to the receptor or antibody binding site. This is usually expressed as an equilibrium constant, Kd. The lower the Kd value, the higher the affinity. This also relates to the concentrations of unlabeled ligand that can be measured in the competitive assay.
Specificity or Cross-reactivity describes how selective an antibody binding site is for a particular ligand, and which structurally-similar ligands might interfere with the assay
Kd: The concentration where 50% of the receptors or antibody binding sites are occupied by radioligand/tracer.
IC50: Concentration of a competing ligand that displaces half of the radioactive ligand. This is the calibration curve mid-point.
Types of assays
Saturation assay
In creating or optimizing your own radioimmunoassay, a saturation experiment is usually performed. This experiment measures binding equilibrium via titration of radioligand (tracer), keeping the amount of receptor or antiserum dilution constant. You can use saturation curves to determine Bmax (binding site expression level) and Kd (binding affinity of ligand-antibody interaction). Choosing a concentration of radiolabeled ligand (tracer) at about 50-60% of the saturation level generally gives the correct tracer mass to use in the assay.
Q. What concentrations of radioligand should I use for my saturation curve?
A. We generally recommend you choose 3-5 concentrations below the estimated Kd, and 3-5 concentrations above the estimated Kd. The highest concentration tested should be ten times the Kd (10 x Kd).
Figure 4 shows the graphic representation of a saturation binding assay, using increasing concentrations of the tritiated radioligand cyclopentyl-1,3-dipropylxanthine with a constant amount (concentration) of binder. In this case, the binder is a receptor membrane at 10 µg per mL. In a radioimmunoassay, the binder would be a specified dilution of antiserum.
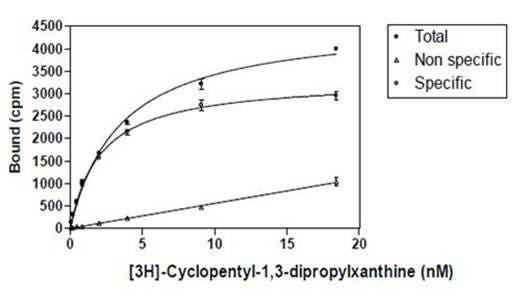
Figure 4. Saturation curve showing binding of 3H-cyclopentyl-1,3-dipropylxanthine to its receptor. Increasing amounts of radioligand are added to a fixed concentration of receptor. Total binding: Increasing concentration of radioligand in absence of cold ligand. Measures both specific binding to receptor, as well as non-specific binding. Non-specific binding: Increasing concentration of radioligand in presence of excess unlabeled ligand. Measures binding of the radioligand to non-receptor or non-antibody components. Specific binding: Total minus non-specific binding. Measures binding to the receptor or antibody, specifically.
Competition assay
Competition assays measure the equilibrium binding of a single concentration of radioligand (tracer) in the presence of various concentrations of competing unlabeled calibration ligand (standard) or sample of unknown concentration.
Protocol for a hypothetical RIA
- Equilibrate all reagents to room temperature and mix before use.
- Label duplicate tubes for total counts, NSB (blank), each standard, and each sample (refer to Table 2 below).
- Place tubes in a suitable test tube rack.
- Incubate overnight (16 - 24 hours) at 2 - 8°C.
- Add 1 mL of cold Precipitating Reagent to all tubes except total counts, and mix.
- Incubate for 20 - 30 minutes at 2 - 8°C, and centrifuge at 2 - 8ºC. for 30 minutes at 1000 - 2000 x g.
- Decant the supernatants from all tubes (except total count tubes) into an appropriate radioactive liquid waste tray.
- Blot the liquid from the rims of the assay tubes on absorbent paper mats for ~ 1 minute.
- Count the radioactivity remaining in the assay tubes (including the Total Count tubes).
Table 2. Tubes for radioimmunoassay protocol. All volumes are in microliters (µL).
| Tube No. | Buffer | Standard | Samples | Tracer | Antibody | |
|---|---|---|---|---|---|---|
| Total Counts | 1-2 | --- | --- | --- | 100 | --- |
| Blank | 3-4 | 200 | --- | --- | 100 | --- |
| "0" Standard | 5-6 | 100 | --- | --- | 100 | 100 |
| Standards | 7-20 | --- | 100 | --- | 100 | 100 |
| Samples | 21,22, etc. | --- | --- | 100 | 100 | 100 |
Q. What concentration of radioligand (tracer) should be selected for competitive binding?
A. The radioligand (tracer) is used at a low concentration, usually at or below its Kd value. If the specific activity is low, concentrations above the Kd value can be used, though the concentration must never be at or higher than saturating concentrations.
Calculations
After counting is complete, the concentration of analyte in the samples is determined by interpolation from a standard curve. The following method is suggested (refer to Table 3 for sample calculations).
A. Average the counts for each set of tube replicates.
B. Calculate the average NET counts for all standards (including the Zero Standard) and samples by subtracting from each the average non-specific binding counts.
C. Determine the normalized percent bound (% B/B0) for each standard and sample as follows:
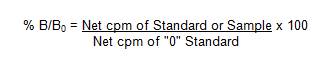
D. Plot % B/B0 for each standard versus the corresponding amounts of unlabeled ligand calibrator added in picograms. See Figure 5 for a typical standard curve using the standard protocol.
E. Determine the amount of analyte in each sample by interpolation from the standard curve. Because the standard curve is expressed as "picograms added", sample values must then be corrected for any dilutions to determine the original concentration in the sample.
NOTE: Any samples with concentrations which are above the range of the standard curve must be diluted with assay buffer and re-assayed. The values obtained are then multiplied by the appropriate dilution factor. Values obtained that are lower than the first calibration standard are suspect.
Table 3. Raw and processed data.
| Tube | No. | CPM | Avg CPM | Net Avg CPM | %B/B0 |
|---|---|---|---|---|---|
| Total Counts | 1 | 14963 | |||
| 2 | 15563 | 15263 | |||
| Blank | 3 | 415 | |||
| 4 | 380 | 398 | |||
| "0" Standard | 5 | 8879 | |||
| 6 | 8673 | 8776 | 8378 | 100 | |
| 2 pg | 7 | 7701 | |||
| 8 | 7939 | 7820 | 7422 | 88.6 | |
| 5 pg | 9 | 6873 | |||
| 10 | 6886 | 6880 | 6482 | 77.4 | |
| 10 pg | 11 | 5632 | |||
| 12 | 5900 | 5766 | 5368 | 64.1 | |
| 25 pg | 13 | 4216 | |||
| 14 | 4256 | 4236 | 3838 | 45.8 | |
| 50 pg | 15 | 3082 | |||
| 16 | 2995 | 3039 | 2641 | 31.5 | |
| 100 pg | 17 | 2257 | |||
| 18 | 2003 | 2130 | 1732 | 20.7 | |
| 200 pg | 19 | 1491 | |||
| 20 | 1661 | 1576 | 1178 | 14.1 | |
| Sample | 21 | 5829 | |||
| 22 | 5919 | 5874 | 5476 | 65.4 |
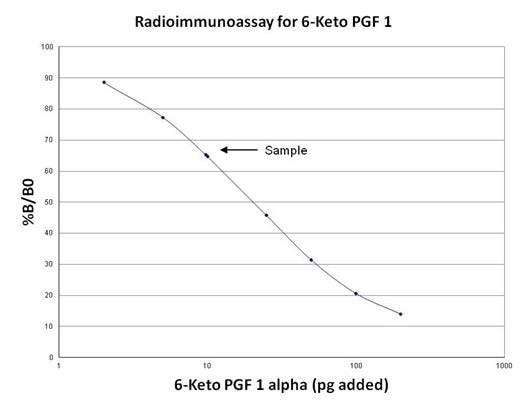
Figure 5. Standard curve for 6-Keto PGF1. The concentration of an unknown sample is interpolated from the curve.
Tips and FAQs
Particularly when using an automated data reduction system, it is essential that you review your raw data. Check that your Total Count tubes, non-specific binding, zero standard binding and calibration curve shape make sense. If any of your assay components are degrading, you will notice by inspecting your data.
- The area of your calibration curve between your zero standard (no analyte standard) binding and your first calibration curve standard will be below the sensitivity of detection. If you need to measure samples below this, you will need to concentrate them, or...
- Another trick that you can try is a delayed addition assay. You can usually increase the sensitivity of detection of the assay by incubating the antibody and the calibration standards/samples for 3-4 hours without the tracer. After this initial incubation, add the tracer, mix the tubes, and incubate overnight. If needed, you could also optimize a second assay with a more-sensitive binder (antibody).
- Try to standardize your incubation times, especially overnight incubations, to prevent inter-assay variation.
- Hydrophobic ligands will generally show higher non-specific binding. Addition of Tween or Triton X-100 detergent may help this.
- Use high purity radioligands/tracers (typically > 90% pure)
- Use highly selective antibodies (with low cross-reactivity to structurally similar compounds).
- Stability - if you will need to use your radioligand over an extended period of time, stability may be a factor. 125I-labeled ligands should generally be used within one to two months of manufacture date. The amount of cpm’s will naturally decrease as the radioisotope decays. Tritiated ligands should usually be used within 3-6 months of manufacture date (however, there are exceptions to this).
References
- Radioimmunoassay. W.M. Hunter in Handbook of Experimental Immunology-Volume 1 Immunochemistry, D.M. Weir (Ed), Blackwell Scientific Publications, London 1978.
- Radioimmunoassay. K.E. Kirkham & W. M. Hunter, Williams & Wilkins Co., Baltimore, MD 1971
- Personal Communications- S. Richard Harris Ph.D, Dupont-NEN Biomedical Products.
- Personal Communications- David Handfield and Scott Keohane.
- Immunology - Dr Janis Kuby - W.H. Freeman & Co., NY 2007
Custom services at Revvity
Revvity offers custom radiochemical services. Visit the link below or contact us.
Radiosynthesis and Labeling Custom Services
For research use only, not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























