Overview
Acute inflammation is an initial biological response of vascular tissues to tissue injury or infection. Several diseases are characterized by acute inflammation, including bacterial and fungal infections, inflammatory bowel disease, Crohn’s disease, kidney, lung and liver diseases, trauma, and various degenerative diseases including arthritis. Acute inflammation is characterized by vascular changes, including vasodilation, increased vascular permeability, edema, and increased blood flow.
The difficulty in studying inflammation in vitro increases the importance of robust and quantitative in vivo techniques to assess progressive inflammation and therapeutic intervention. In vivo imaging targets for acute inflammation include proteases and other mediators involved in the inflammation process; leukocytes, monocytes and macrophages that migrate to the injured tissue; macromolecules that accumulate in inflamed tissues due to increased vascular permeability; and the vasculature itself.
The ability to non-invasively and quantitatively study the underlying biology of inflammatory immune responses in real time is critical in the development and monitoring of anti-inflammatory therapies. Understanding the pathophysiology of inflammatory disease processes at the cellular and biochemical level is a major challenge in basic research and drug development.
Products for imaging acute inflammation
Our pre-clinical imaging probes fall into three main categories: Activatable, meaning they are optically silent until cleaved by a protease of interest; Targeted, meaning they bind to a receptor or other cellular membrane expressed in the disease of interest; or Vascular, meaning they localize to vasculature and are used to assess changes in vascular permeability. All three types of probes can be used to assess acute inflammation in vivo. We also offer the cell labeling dye (IVISense™ fluorescent cell labeling dyes, see table below). Probe properties, protocols and instrument settings, can be found by clicking on the probe links below. All our probes are compatible with FMT and IVIS imaging systems, provided that your system has the capabilities to image the probes at the proper wavelengths.
| Probe/Dye | Probe/Dye Type | Probe/Dye Mechanism | Available Wavelengths and optimal in vivo imaging time (post-injection) | Route of metabolism/ background tissue(s) | Validated imaging methods |
|---|---|---|---|---|---|
| IVISense Cat B FAST | Activatable, fluorescent | Detects cathepsin B activity, a lysosomal protease released by monocytes and macrophages to target inflammatory products, helping to degrade the cell matrix, cell debris and pathogens. Cathepsin B activity has been positively correlated with the degree of inflammation and neutrophil recruitment. | 680: 6-24 hours 750: 6-24 hours |
Salivary glands, liver, kidneys | In vivo, flow cyometry, in vitro cell microscopy, frozen tissue labeling |
| IVISense MMP | Activatable, fluorescent | Detects the activity of matrix metallo- proteases 2, 3, 7, 9, 12 and 13 (MMP2, MMP3, MMP7, MMP9, MMP12, MMP13), proteases secreted in response to acute inflammation. MMPs mediate degradation of essentially all components of the extracellular matrix. | 645 FAST*: 24 h (6-24 h) 680: 24 h (24-36 h) 750 FAST*: 12 h (12-24 h) |
Liver, kidneys (645 FAST, 750 FAST) Liver only (680) |
In vivo |
| IVISense Neutrophil Elastase FAST | Activatable, fluorescent | Measures the activity of neutrophil elastase, which is secreted by neutrophils and macrophages during an inflammatory response. | 680: 3-6 h | Bladder, liver, intestines | In vivo, frozen tissue labeling |
| IVISense Pan Cathepsin | Activatable, fluorescent | Detects cathepsin B, L, S and plasmin, which are released by immune cells in response to inflammatory mediators | 680: 24 h (24-48 h) 750: 24 h 750 FAST*: 6-24 h |
Liver (680) Low liver (750) Low liver, bladder (750 FAST) |
In vivo, flow cytometry, in vitro cell microscopy |
| Targeted, chemi- luminescent | Detects myelo- peroxidase activity, which is released by activated phagocytes in both chronic and acute inflammation | Emits at 425nm, 10 min post-injection, 5 min BLI acquisition time, internal fluorescent control imaged at 745 nm excitation and 800 nm emission 1-5 sec |
Kidney |
In vivo | |
| IVISense Folate Receptor | Targeted, fluorescent | Binds to folate receptors, which are overexpressed in activated macrophages | 680: 6 h (6-24 h) | Kidney | In vivo, flow cytometry, in vitro cell microscopy, frozen tissue labeling |
| IVISense Vascular | Vascular, fluorescent | Will accumulate in areas of vascular leakage associated with tumorigenesis and inflammation; used to assess vascular leak | 680: 24 h 750: 24 h |
Low liver, lung | In vivo |
| IVISense Vascular NP | Vascular, fluorescent | Nanoparticle for imaging vascularity, long PK profile, 20 hour half-life in plasma | 680: 24 h 750: 24 h |
Long term tissue accumulation | In vivo |
| IVISense Edema | Vascular, fluorescent | Low MW albumin-binding probe that clears from the blood and tissue rapidly; used to image acute edema | 680: 3 h (1-3 h) | Bladder | In vivo |
| IVISense Fluorescent Cell Labeling Dyes | Labeling, fluorescent | Cell labeling dye; can be used to label macrophages in vitro and track them in vivo to monitor recruitment and localization in acute inflammation | 680: variable hours | In vivo, flow cytometry, in vitro cell microscopy |
- Jochum, M., Machleidt, W. & Fritz, H. Proteolysis-induced pathomechanisms in acute inflammation and related therapeutic approaches. Agents Actions Suppl.42, 51–69 (1993).
- Warner, R. L. et al. Matrix metalloproteinases in acute inflammation: induction of MMP-3 and MMP-9 in fibroblasts and epithelial cells following exposure to pro-inflammatory mediators in vitro. Exp. Mol. Pathol.76, 189–195 (2004).
- Korkmaz, B., Horwitz, M. S., Jenne, D. E. & Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin Gas therapeutic targets in human diseases. Pharmacol. Rev.62, 726–759 (2010).
Choosing the right probe
When choosing a probe for acute inflammation studies, several factors should be taken into consideration:
- Fluorescent vs. chemiluminscent probes: Be sure to choose the proper probe for your instrumentation. Some imagers can measure both chemiluminescence and fluorescence, while others can only measure one or the other.
- Dye excitation/emission wavelength: Some fluorescent probes are available with excitation wavelengths that range from 645 nm to 800 nm. Be sure to pick a wavelength that is appropriate for both your instrument and application. Most microscopes filters are not suitable for wavelengths above 680 nm. However deep tissue imaging typically has less background fluorescence and works better with longer wavelengths (750 nm- 800 nm).
- Probe clearance time is typically faster for activatable probes (particularly the FAST™ platform) which allows for re-injection after shorter time periods.
- Probe specificity: For targeted and activatable probes, it is important to remember that the protein or enzyme that the probe is targeting needs to be present and well expressed in your mouse model.
- In vivo distribution: Some probes might accumulate in organs at timepoints which could interfere with your study. Be sure to check the biodistribution profile of each probe of interest.
- Type of imaging required for your study: Vascular probes for example, while useful in assessing vascularity changes in the mouse, do not make good probes for in vitro cell imaging or tissue analysis. If these types of analyses are desired, targeted or activatable probes might be a better choice for you.
For more information about probes to study chronic inflammatory models, please visit the Arthritis, Atherosclerosis, Bacterial Infection, or Pulmonary Inflammation sections.
Mouse model of acute inflammation
Carrageenan paw edema
A model commonly used to study acute inflammation in mice is carrageenan-induced paw edema (CPE). Inflammation and swelling are induced by injecting carrageenan into the footpad of the mouse. Carrageenan elicits an inflammatory response by stimulating pro-inflammatory probes such as neutrophils which migrate to the site of infection, resulting in edema within the first 3-4 hours. At later timepoints (~24 hours), edema decreases as macrophages infiltrate the site of injection. For a protocol on how to prepare carrageenan for mouse injections, click here.
In the examples below, Revvity’s NIR imaging probes can be injected either at 3 or 24 hours after the administration of carrageenan to determine the extent of edema and cellular inflammation non-invasively. Unlike traditional methods (i.e. caliper measurements of footpad thickness) which effectively represent the magnitude of the inflammatory response, these fluorescent probes provide quantification of specific biological processes—such as vascular leak associated with edema.

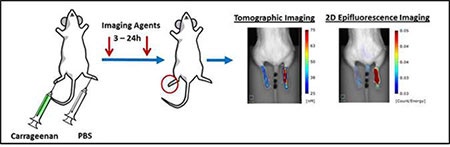
Figure 1: Carrageenan is injected into the right hindpaw of a mouse. Vascular imaging probes are administered 3-24 hours post carrageenan injection. Topographic imaging using Revvity’s FMT system allows quantitative detection through tissues, while 2D epifluorescence primarily measures surface weighted fluorescence in the outer mm of the tissue.
In Figure 2 below, paw thickness measurements at both early (2 hours) and late (24 hours) time points are compared to in vivo quantification of the vascular imaging probe IVISense Vascular and the activatable imaging probes IVISense Neutrophil Elastase 680 FAST and IVISense CAT B 680 FAST.
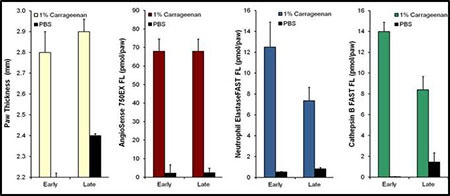
Figure 2: Comparison of Paw Thickness Measurements to Imaging Probes Carrageenan Paw Edema. Paw thickness, vascular leak (IVISense Vascular), neutrophil elastase activity (IVISense Neutrophil Elastase 680 FAST), and cathepsin activity (IVISense CAT B 680 FAST) were assessed in mice that were injected with 1% carrageenan at 2 hours (early) and 24 hours (late) post-CG injection.
In Figure 3, IVISense Vascular (formerly AngioSense) is used to assess vascular leak in the presence or absence of the anti-inflammatory steroid dexamethasone.
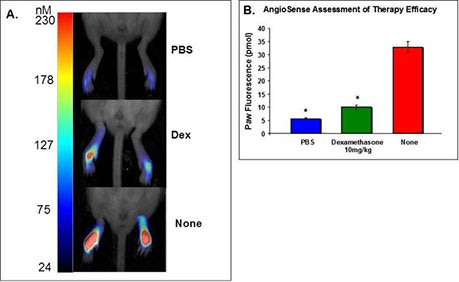
Figure 3: Assessment of Dexamethasone Therapy. Mouse hindpaws were injected with PBS, 10 mg/kg dexamathosone + 1% carrageenan, or 1% carrageenan alone. A) Tomographic images of three conditions. The fluorescent signal is represented as a maximum intensity projection (identical gains set for each mouse) of the 3D regions of interest established around each paw with quantitative color bars to represent fluorescence concentration (nM). B) Quantification of paw fluorescence for each of the three conditions. As expected, the steroid dexamethasone significantly inhibited the vascular response at 3 hours.
Application notes and posters
- Application Note: A method of NIR fluorescent cell-labeling for in vivo cell tracking
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance or use information. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.




























