Overview
Pulmonary inflammation is involved in diseases such as asthma and chronic obstructive pulmonary disease (COPD). The ability to visualize molecular and cellular regulators of pulmonary inflammation in relevant animal models can aid in the understanding of disease mechanisms and the development of effective therapeutics of pulmonary disease. Revvity offers IVISense™ near-infrared fluorescent probes for the noninvasive in vivo imaging and quantitation of pulmonary inflammation, as well as bioluminescent IVISbrite™ bioluminescent tumor cell lines for studying lung cancer and lung metastasis.
Products for imaging pulmonary inflammation
| Probe | Probe Type | Probe Mechanism | Available Wavelengths and optimal in vivo imaging time (post-injection) | Route of metabolism/ background tissue(s) | Validated imaging methods |
|---|---|---|---|---|---|
| IVISense Neutrophil Elastase FAST |
Activatable; fluorescent | Produces fluorescent signal after cleavage by neutrophil elastase mainly produced by neutrophils in sites of inflammation; may be used to monitor NE activity and the efficacy of therapeutic treatment in research animal models of acute lung injury, acute respiratory distress syndrome, emphysema/chronic obstructive pulmonary disease, cystic fibrosis, chronic wound healing, rheumatoid arthritis, atherosclerosis, and infectious diseases (such as septicemia and pneumonia). | 680: 3-6 h | Bladder, liver, intestines | In vivo, frozen tissue labeling |
| IVISense Pan Cathepsin | Activatable; fluorescent | Fluoresces only when activated by disease-related proteases such as cathepsins; allows the detection and quantitation of protease activity associated with eosinophils that are recruited to the lung upon inhalation of triggering allergens in asthma, in small animal models of LPS-induced pulmonary inflammation in COPD, and abnormal increase in protease activity associated with tumor aggression and growth in small animal models of lung cancer and lung metastasis | 680: 24 h (24-48 h) 750: 24 h 750 FAST*: 6-24 h |
Liver (680) Low liver (750) Low liver, bladder (750 FAST) |
In vivo/ex vivo; flow cytometry; in vitro microscopy |
| IVISense MMP | Activatable; fluorescent | Can be used to detect increased MMP activity in eosinophils in small animal models of pulmonary eosinophilia | 645 FAST*: 24 h (6-24 h) 680: 24 h (24-36 h) 750 FAST*: 12 h (12-24 h) |
Liver, kidneys (645 FAST, 750 FAST) Liver only (680) |
In vivo |
| IVISense Vascular | Vascular; fluorescent | Can be used to measure vascular leak in the lungs due to pulmonary edema | 680 nm (24 hours), 750 nm (24 hours) | Low liver lung | In vivo/ex vivo; flow cytometry; in vitro microscopy |
| IVISbrite Tumor Cell Lines | Bioluminescence or dual bioluminescence/ fluorescence | Bioluminescent oncology cell lines (also known as IVISbrite™ cell lines) are stably transfected with a luciferase (luc or luc2) reporter gene that allows you to visualize the growth of the cells in vivo. The cell lines are injected into an appropriate mouse model to monitor early tumor development, monitor tumor growth and metastases, quantify tumor burden in a whole animal model, and follow therapeutic responses non-invasively. | In vivo/ex vivo (dual) |
Choosing the right probe
When choosing a probe for angiogenesis studies, several factors should be taken into consideration:
- Fluorescent vs. bioluminscent probes: Be sure to choose the proper probe for your instrumentation. Some imagers can measure both bioluminescence and fluorescence, while others can only measure one or the other.
- Dye excitation/emission wavelength: Some fluorescent Probes are available with excitation wavelengths that range from 645 nm to 800 nm. Be sure to pick a wavelength that is appropriate for both your instrument and application. Most microscopes filters are not suitable for wavelengths above 680 nm. However deep tissue imaging typically has less background fluorescence and works better with longer wavelengths (750 nm- 800 nm).
- Probe clearance time is typically faster for activatable Probes, which allows for re-injection after shorter time periods.
- Probe specificity: For targeted and activatable Probes, it is important to remember that the protein or enzyme that the probe is targeting needs to be present and well expressed in your mouse model.
- In vivo distribution: Some Probes might accumulate in organs at timepoints which could interfere with your study. Be sure to check the biodistribution profile of each probe of interest.
- Type of imaging required for your study: Vascular Probes for example, while useful in assessing vascularity changes in the mouse, do not make good Probes for in vitro cell imaging or tissue analysis. If these types of analyses are desired, targeted or activatable Probes might be a better choice for you.
View more general information on mouse handling and injection procedures.
For information on lung cancer and metastasis, please visit our Oncology application section.
Mouse models of pulmonary information
Mouse model for Asthma
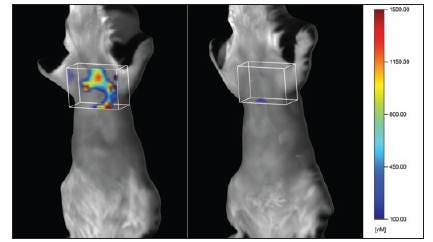
Asthma can be induced in female BALB/c mice by provoking specific immunity to ovalbumin in the lung.1 To do this, we immunized 6- to 8-week-old BALB/c mice on day 0 and day 14 by intraperitoneal injections of 50 μg of ovalbumin combined with 2 g of aluminum hydroxide as an adjuvant to enhance the allergic response. Between day 21 and day 24, mice received daily intranasal challenges with 100 μg of ovalbumin solubilized in PBS (pH 7.4) to focus an ovalbumin specific allergic response within the airways. The allergic response to ovalbumin induces cytokines and immune factors typical of those in human asthma (for example, interleukin (IL)-4, histamine and IgE), a large influx of eosinophils and changes in airway hyperreactivity. To quantitate the inflammation associated with asthma progression, we injected the mice with 5 nmol of IVISense Pan Cathepsin 680 probe at day 24, 4 hours after the final intranasal administration of ovalbumin.

Figure 1. Noninvasive tomographic imaging of disease-associated protease activity in ovalbumin-induced mouse asthma. The asthmatic mouse (left) shows broad distribution of protease-activated IVISense Pan Cathepsin probe fluorescence in the lung, with little fluorescence seen in the lung region of a control mouse (right). The box marks the region of interest established for analysis.
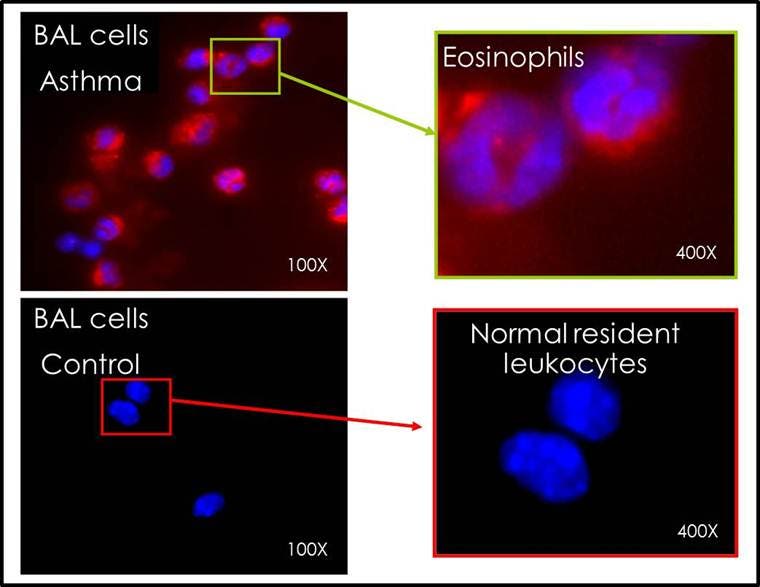
Figure 2. Tissue sections from the lungs of asthmatic mice demonstrated multiple areas of fluorescence localized to the lung parenchyma as compared to the minimal fluorescence detectable in the control lungs.
Mouse model for COPD
Experimental Animals: Specific pathogen-free female BALB/c mice (6-8 weeks of age, 18–20 g) were obtained from Charles River (Wilmington, MA) and housed in a controlled environment (72°F; 12:12-h light-dark cycle) under specific-pathogen free conditions with water and food provided ad libitum.
LPS Inflammation Protocol: LPS-induced lung inflammation is a simple model for cellular processes occurring in Chronic Obstructive Pulmonary Disease (COPD). On day 0, mice received intranasal injections of either 100 μg LPS solubilized in phosphate buffered saline (PBS) or PBS alone. Diseased and control mice were injected with IVISense Pan Cathepsin 680 or IVISense Vascular 680 on day 1, 4 hrs after the intranasal administration of LPS. Mice were anesthetized, depilated to minimize interference with fluorescent signal, and imaged at 7 or 24 hrs, for IVISense Vascular 680 and IVISense Pan Cathepsin 680, respectively.
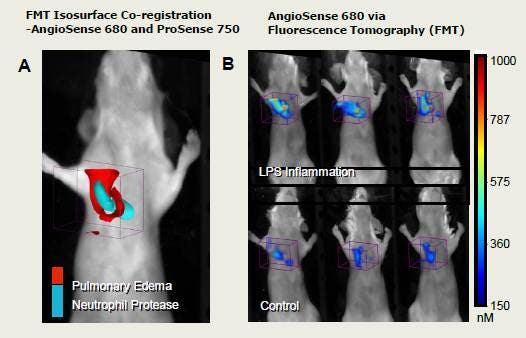
Figure 3. To assess the existence of edema caused by the inflammation of LPS in the airways, we used FMT imaging in combination with a vascular imaging probe, IVISense Vascular 680 (formerly AngioSense). We performed multiplex imaging to compare the pattern of distribution (represented as colored, volumetric surface renderings) of IVISense Vascular 680 to the highly characterized distribution that we have established with IVISense Pan Cathepsin 750 (formerly ProSense) imaging of neutrophilia (A). As expected, the sites of neutrophilia were spatially distinct from regions showing leakage of the vascular probe due to pulmonary edema; edema signal surrounded regions of more centralized neutrophilia. Representative mice are shown (n = 5 per group) to portray the consistent edema signal induced by LPS as compared to background signal in control animals (B). Fluorescence molecular tomography quantified a 5-fold increase in total IVISense Vascular 680 fluorescence in the lungs of LPS mice (> 130 pmol/lung) indicating increased vascular leak in the inflamed lungs as compared to those of control mice (< 20-30 pmol/lung and p<0.003).
Application notes and posters
Asthma
- Poster: Non-Invasive Quantitative Tomography of Disease Progression and Therapeutic Response in a Mouse Model of Asthma In Vivo
- Application Note: Non-Invasive, in vivo quantification of asthma severity using fluorescence molecular tomography (FMT)
COPD
- Poster: Non-Invasive near-Infrared Fluorescence Quantitative Tomography (FMT) of the Effects of PDE4 Inhibitor Therapy in an LPS Murine Model of COPD In Vivo
The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance on injection techniques or other applicable needs. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information. The information is provided on an "as is" basis without warranties of any kind. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.





































