Overview
Atherosclerosis is a disease of the arterial blood vessels (arteries) characterized by the build-up of waxy plaques on the inside of blood vessels (in the inner, endothelial layer). It is a condition in which the artery wall thickens as a result of the buildup of fats and cholesterol in and on artery walls (plaques), which can restrict blood flow. These plaques can burst, triggering a blood clot (thrombosis) or, if the flow of blood is completely blocked, they could cause a heart attack via the coronary arteries or a stroke if the carotid arteries are affected.
Atherosclerosis is characterized by a chronic inflammatory response in the walls of arteries caused largely by the accumulation of macrophage white blood cells and promoted by low-density lipoproteins (LDL) without adequate removal of fats and cholesterol from the macrophages by functional high-density lipoproteins (HDL). Substances or processes that cause atherosclerosis are called atherogenic. It is caused by the formation of multiple plaques within the arteries. It is a chronic disease that commonly remains asymptomatic for decades.
The initial stages of atherosclerosis are characterized by infiltration of macrophages and growth of isolated foam cells. This leads to the development of “fatty streaks” in the arterial wall (small sub-endothelial deposits of monocyte-derived macrophages). Promoted by LDLs and inadequate HDLs this tissue insult drives an inflammatory cascade that recruits additional macrophages to the artery wall. The macrophages, which ingest oxidized LDL, slowly turn into large “foam cell” macrophages, containing numerous internal cytoplasmic vesicles with high lipid content. Death of these foam cells further propagates the inflammatory process.
ApoE-deficient (apoE-/-) mice model many aspects of the pathogenesis of human disease. When on high cholesterol diets, these mice develop sever hypercholesterolemia and lesions that progress from fatty streaks to fibrous plaques in lesion-prone areas throughout the aorta.
Products for imaging atherosclerosis
| Probe/Dye | Probe/Dye Type | Probe/Dye Mechanism | Available Wavelengths and optimal in vivo imaging time (post-injection) | Route of metabolism/ background tissue(s) | Validated imaging methods* |
|---|---|---|---|---|---|
| IVISense™ Pan Cathepsin & IVISense Pan Cathepsin FAST | Activatable; fluorescence | Broadly detects cathepsin activity which is upregulated in inflammatory cells such as foam cells. It is taken up in the lysosomes of inflammatory cells associated with atherosclerosis and cleaved by multiple cathepsins, activating fluorescent signal. | 680 nm (24-48 hours) 750 nm (FAST) (24 hours) 750 nm (6-24 hours) |
Liver (680 nm) Low liver/intestine (750 nm FAST) Low liver/bladder (750 nm) |
In vivo/Ex vivo , Flow cytometry, In vitro microscopy |
| IVISense Cat B FAST | Activatable; fluorescence | Taken up into the lysosomes of atherosclerosis-associated inflammatory cells and is selectively activated by cathepsin B. | 680 nm (36 h) 750 nm (36 h) |
Salivary glands, liver, kidneys | In vivo/Ex vivo , Flow cytometry, In vitro microscopy |
| IVISense Cat K FAST | Activatable; fluorescence | Selectively activated by osteoclast-associated cathepsin K at sites of bone resorption or tissue calcification. This may be used in later stages of atherosclerosis development when calcification occurs. | 680 nm (6-24 h) | Kidney, liver | In vivo/Ex vivo , Flow cytometry, In vitro microscopy |
| IVISense MMP and IVISense MMP FAST | Activatable; fluorescence | MMP-2 and MMP-9 are considered important biomarkers in the formation of a vulnerable plaque. | 645 nm (6-24 h) 680 nm (24-36 h) 750 nm (12-24 h) |
Liver and kidneys (645 nm and 750 nm) Liver (680 nm) |
In vivo/ex vivo |
| IVISense Annexin-V | Targeted; fluorescence | Targeted probe that labels cells that are undergoing the early stages of apoptosis. It is useful in visualizing the progression of atherosclerosis as cellular necrosis occurs increasingly during later stages of the disease. | 750 nm (2 h) | Kidneys (high), liver | In vivo/Ex vivo , Flow cytometry, In vitro microscopy |
| IVISense Integrin Receptor | Targeted; fluorescence | Small molecule αvß3 integrin antagonist that contains a NIR fluorophore reporter. This targeted probe detects increased integrin expression associated with neovasculature, tumors, and some inflammatory cells associated with atherosclerosis. | 645 nm (48 h) 680 nm (24 h) 750 nm (24 h) |
Bladder/kidneys (645 nm) Kidneys/intestine (680 nm) Kidneys (750 nm) |
In vivo/Ex vivo , Flow cytometry, In vitro microscopy |
| IVISense Osteo | Targeted; fluorescence | Bisphosphonate imaging probe that enables imaging of bone growth and resorption; can be used to measure bone cancer metastasis. | 680 nm (3-24 h) 750 nm (3-24 h) 800 nm (3-24 h) |
Bladder | In vivo |
| IVISense Vascular NP | Vascular; fluorescence | Pegylated fluorescent nanoparticles that remain localized in the vasculature for extended periods of time and enable imaging of blood vessels and angiogenesis | 680 nm (0 - 4 hours) | Long term tissue accumulation | In vivo |
Choosing the right probe
When choosing a probe for angiogenesis studies, several factors should be taken into consideration:
- Fluorescent vs. Bioluminscent probes: Be sure to choose the proper probe for your instrumentation. Some imagers can measure both bioluminescence and fluorescence, while others can only measure one or the other.
- Dye excitation/emission wavelength: Some fluorescent probes are available with excitation wavelengths that range from 645 nm to 800 nm. Be sure to pick a wavelength that is appropriate for both your instrument and application. Most microscopes filters are not suitable for wavelengths above 680 nm. However deep tissue imaging typically has less background fluorescence and works better with longer wavelengths (750 nm- 800 nm).
- Probe clearance time is typically faster for activatable probes, which allows for re-injection after shorter time periods.
- Probe specificity: For targeted and activatable probes, it is important to remember that the protein or enzyme that the probe is targeting needs to be present and well expressed in your mouse model.
- In vivo distribution: Some probes might accumulate in organs at timepoints which could interfere with your study. Be sure to check the biodistribution profile of each probe of interest.
- Type of imaging required for your study: Vascular probes for example, while useful in assessing vascularity changes in the mouse, do not make good probes for in vitro cell imaging or tissue analysis. If these types of analyses are desired, targeted or activatable probes might be a better choice for you.
Mouse models for artheroschlerosis
Experimental Animals. Specific pathogen-free female Apolipoprotein (apo) E-deficient mice (6-8 weeks of age, 18–20 g) were obtained from The Jackson Labs (Bangor, ME) and housed in a controlled environment (72°F; 12:12-h light-dark cycle) under specific pathogen-free conditions. Mice homozygous for the Apoetm1Unc mutation show a marked increase in total plasma cholesterol levels that are unaffected by age or sex. Fatty streaks in the proximal aorta are found at 3 months of age, and the lesions increase with age. To accelerate the onset of disease, these mice were fed a 0.2% high cholesterol diet (Western type No. 88137, Harlan Laboratories, Madison WI) for assessment at different timepoints relative to the start of feeding (from 15 to 30 weeks on diet). Age-matched C57BL/6 mice were used as wild-type (WT) controls for all studies and were maintained on standard chow and water. All experiments were performed in accordance with VisEn Medical IACUC guidelines for animal care and use.
NIR Fluorescent Imaging Probes for the Detection of Atherosclerosis. A suite of biomarker-specific imaging probes (Table 1) were used to detect atherosclerosis severity, progression, and response to therapy. Individual probes targeted at specific biomarkers of atherosclerotic disease were imaged using fluorescence quantitative tomography to quantify protease activity or tissue integrin levels in vivo. Each of the probes were administered intravenously in all imaging studies. The typical adult mouse dose is 2 nmoles in 150 μL saline, but for these studies a higher dose of 8 nmoles/mouse was used to enhance signal within the very small anatomical region of the aortic arch. Imaging of IVISense Pan Cathepsin and IVISense Integrin Receptor probes was performed 24h following injection of the probe, whereas optimal imaging timepoints for the FAST probes (Cat K and Cat B FAST) was 6h.

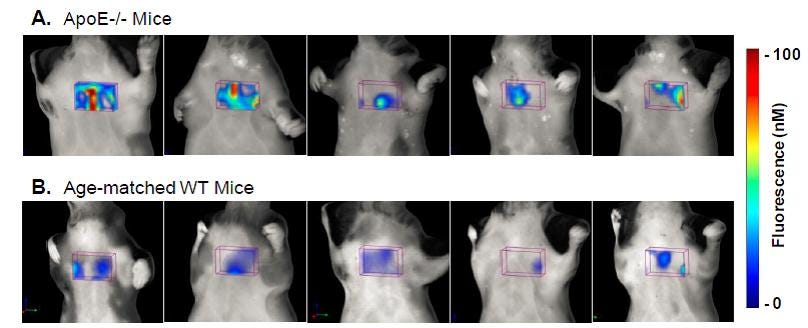
Figure 1. Fluroescence tomographic images of IVISense Pan Cathepsin 750 in ApoE-/- and WT Mice. A. Images from 5 representative apoE-/- mice maintained on high-cholesterol diets for 30 weeks were generated 2500 24h after IVISense Pan Cathepsin 750 was injected (8 nmol per mouse). B. Images from control WT mice on normal chow. Fluorescence represented is from the aortic arch ROI only, with other non-heart region sources of fluorescence excluded for clarity. No fluorescence thresholding was applied to the tomographic datasets. Images show that there is a range of signal within the upper heart region ROI in ApoE-/- mice, but signal is much lower in wild-type (WT) control C57BL/6 mice.
Application notes and posters
- Application note: Non-Invasive Quantitative In Vivo Imaging of Atherosclerosis Disease Progression and Treatment Response in ApoE Deficient Mice using Fluorescence Molecular Tomography and NIR Fluorescent Pre-clinical Imaging Probes
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.




























