Overview
Vertebral skeleton development and maintenance depends upon a balance between osteoblast and osteoclast activity. Osteoblasts are cells that are important in the formation of bone, whereas osteoclasts are cells that resorb bone. A change in the balance between these two cell types is characteristic of numerous disease states. These diseases include arthritis, osteoporosis, and cancer metastases. Hydroxyapatite (HA) is a mineral form of calcium apatite and is the major mineral product of osteoblasts. Therefore, HA levels are a good biomarker for osteoblast activity. In addition, abnormal accumulation of HA can be indicative of a disease state. IVISense™ Osteo (formerly OsteoSense) fluorescent probes were designed to bind with high affinity to HA both in vitro and in vivo. Since hydroxyapatite (HA) is known to bind pyrophosphonates and phosphonates as well as synthetic bisphosphonates with high affinity, IVISense Osteo probes were designed as bisphosphonate imaging probes. Specifically, IVISense Osteo probes can be used to image areas of microcalcifications, bone remodeling and enables imaging of bone growth and resorption.

Products and catalog numbers
| Product | Catalog Number | Ex/Em wavelength (nm) | Molecular weight (g/mol) | Validated Experiments | Applications |
|---|---|---|---|---|---|
| IVISense Osteo 680 | NEV10020EX | 668/687 | 1470.5 | In vivo | Oncology Arthritis Osteoporosis |
| IVISense Osteo 750 | NEV10053EX | 749/770 | 1101.1 | In vivo | Oncology Arthritis Osteoporosis |
| IVISense Osteo 800 | NEV11105 | 780/805 | 1281 | In vivo | Oncology Arthritis Osteoporosis |
Using IVISense Osteo probe for in vivo studies
IVISense Osteo probes can be used to measure the effects of therapeutic stimulation of bone growth and can be used to characterize bone remodeling associated with animal models of arthritis.
- IVISense Osteo 680 and IVISense Osteo 750 are administered via tail vein injection and imaged 24 hours post tail vein injection.
- IVISense Osteo 800 is administered via intravenous injection and imaged 24 hours post injection.
See instructions for setting up an in vivo mouse imaging experiment with IVISense Osteo fluorescent probes:
- IVISense Osteo 680
- IVISense Osteo 750
- IVISense Osteo 800
| Product | Route of Injection | Mouse Dose (25 g) | Rat Dose (250 g) | Blood t 1/2 | Tissue t 1/2 | Optimal imaging time | Optimal Re-injection Time (complete clearance) | Route of Metabolism/ background tissue | FMT & IVIS settings |
|---|---|---|---|---|---|---|---|---|---|
| IVISense Osteo 680 | IV (IP) | 2 nmol | 6 nmol | 5-10 min | ~ 30 days | 3-24 h | Preimage subtraction | Bladder | FMT 680/700 IVIS 675/720 |
| IVISense Osteo 750 | IV (IP) | 4 nmol | 12 nmol | 5-10 min | 7-10 days | 3-24 h | Preimage subtraction | Bladder | FMT 750/770 IVIS 745/800 |
| IVISense Osteo 800 | IV (IP) | 2 nmol | 6 nmol | 5-10 min | 7-10 days | 3-24 h | Preimage subtraction | Bladder | FMT 790/810 |
In Vivo Imaging Applications:
Imaging Active Bone Remodeling:
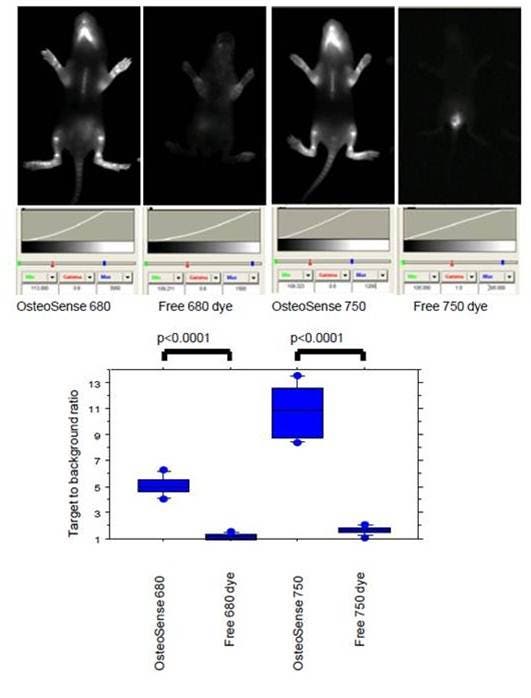
Figure 1: IVISense Osteo 680 (formerly OsteoSense 680), IVISense Osteo 750 (formerly OsteoSense 750) or free dyes (0.5 nmoles) were injected intraperitoneally in 1 week-old Balb/c mice. Imaging was performed 24 hours later on a planar Kodak 2000 MM system. The lower chart shows the target to background ratio. This was defined as the fluorescent signal (relative fluorescent units) divided by the adjacent tissue background signal.
Imaging Bone:
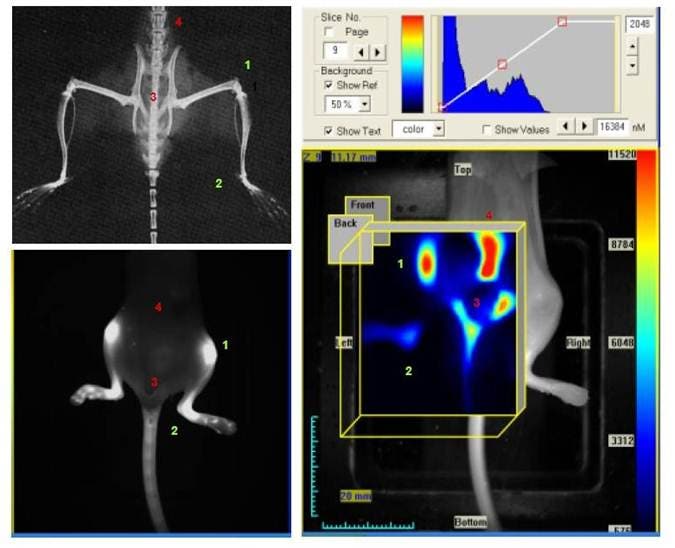
Figure 2: X-ray (upper left), reflectance (lower left) and FMT images of a mouse (right). The same mouse was imaged on an FMT system in reflectance and tomographic modes 24 hours after injection with IVISense Osteo 680 (formerly OsteoSense 680). The numbers indicate various bone structures: 1) knee; 2) ankle; 3) pelvis; 4) vertebrae. The fluorescence of the IVISense Osteo probes on the FMT indicates areas of bone renewal. Using the tomographic mode (right), the FMT allows visualization of deeper pelvic and vertebral structures, which are not visible on reflectance.
Imaging Arthritis:
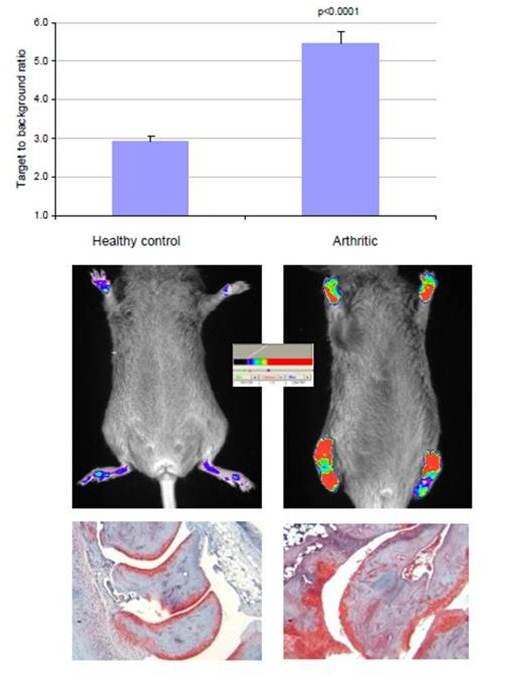
Figure 3: Arthritis was induced in DBA/1J mice by first injecting of bovine collagen (1 mg/ml) in Complete Freund’s Adjuvant (4 mg/ml M. tuberculosis) subcutaneously at the base of the tail. Then, twenty-one days later, the mice were boosted with a subcutaneous injection of collagen (1 mg/ml) in Incomplete Freund’s Adjuvant. The thickness of the paw was measured using a digital Vernier caliper. Imaging was performed in arthritic and healthy (non-induced) mice by injection of IVISense Osteo 680 (2 nmoles) and imaging on a planar Kodak 2000 MM system 24 hours later. Regions of interests within the paws are highlighted. The target-to-background ratio was defined as the specific fluorescent signal (relative fluorescent units) divided by the adjacent tissue background signal.
Simulated Bone Growth:
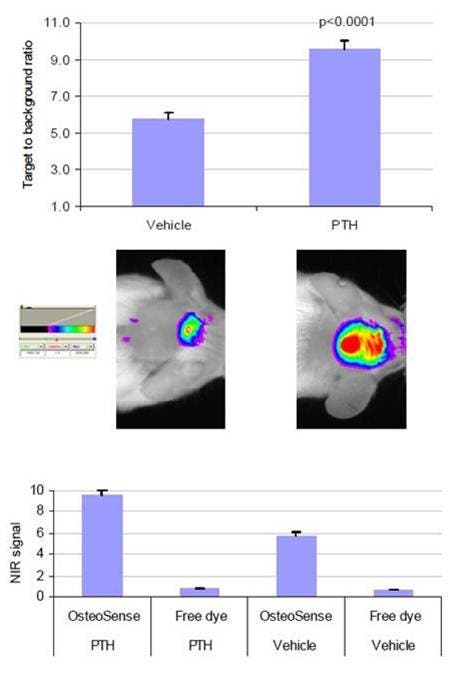
Figure 4: Injections of human PTH (1–34) were delivered twice daily subcutaneously onto the calvaria of CF-1 mice for 12 days to induce Calvarial bone growth. The control mice were injected with only the vehicle. Twenty-four hours after injection of IVISense Osteo 680 (formerly OsteoSense 680), imaging was performed. The visible calvaria region shows regions of interest. The target to background ratio was defined as the fluorescent signal (relative fluorescent units) divided by the adjacent tissue background signal.
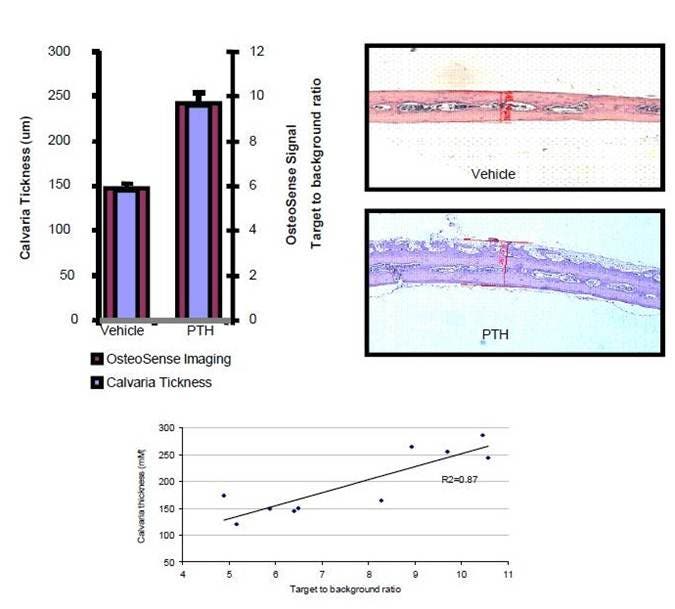
Figure 5: Immediately following imaging (Figure 5), the mice were sacrificed, calvarias dissected, fixed in buffered formalin and decalcified. The sections were stained with Hematoxylin and Eosin, and the calvaria thickness was measured using a Zeiss Axioscope microscope and Axiovision software. From this data, it is clear that IVISense Osteo 680 (formerly OsteoSense 680) can be used to image and quantify stimulated bone growth.
Application notes and posters
- Poster: Imaging of Cathepsin K activity in rodent models of bone turnover and soft tissue calcification
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance or use information. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.




























