Overview
Programmed cell death, or apoptosis, is an important component of various cellular processes. These include embryonic development, hormone-dependent atrophy, normal mature cell turnover, proper development and functioning of the immune system. Apoptosis can also occur as a defense mechanism, such as in immune responses or when cells are damaged by disease or chemical agents. However, many human diseases and conditions can be linked to an increase or decrease in the normal levels of apoptosis. For example, neurodegenerative diseases, ischemic damage, autoimmune disorders and many types of cancer have been attributed to inappropriate levels of apoptosis. Apoptotic cells can be recognized by defined morphological changes such as cellular shrinking, chromatin condensation, and eventually cell fragmentation into small apoptotic bodies.
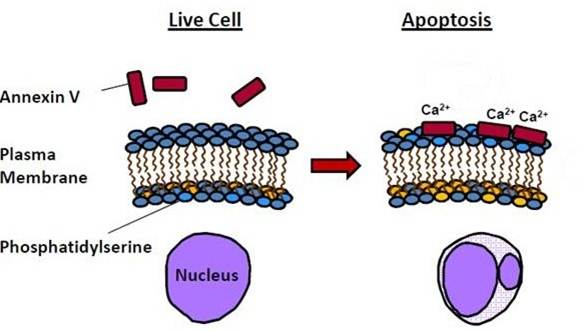
One of the early events in apoptosis is the translocation of phosphatidylserine (PS) from inside the cell to the outer membrane. This change provides a signal to macrophages favoring phagocytosis over the inflammatory response. The PS translocation in apoptosis can be detected by using labeled annexin V. Annexin V is a 36 kDa human protein that binds specifically, in a calcium-dependent manner, with nanomolar affinity to externalized PS.
IVISense™ Annexin-V 750 (formerly Annexin-Vivo) was developed by conjugating annexin V with a NIR fluorophore. This probe has been developed to enable visualization and quantification of the membrane-bound phospholipid, PS, exposed in the outer leaflet of the cell membrane lipid bilayer during the early stages of apoptosis. It is an in vivo imaging tool used in animal models to investigate a variety of pathological conditions (including cancer, stroke, atherosclerosis, myocardial ischemia, and liver toxicity), thereby facilitating the discovery, efficacy evaluation and characterization of novel therapeutics.

Figure 1: Diagram of annexin V function in apoptosis. During apoptosis, phosphatidylserine (PS) is translocated from the inside of the cell to the outer membrane. Annexin V can then bind PS in a calcium-dependent manner.
Products and catalog numbers
| Product | Catalog Number | Ex/Em wavelength (nm) |
Molecular weight (g/mol) | Validated Experiments | Applications |
|---|---|---|---|---|---|
| IVISense Annexin-V 750 | NEV11053 | 755/772 | 35,000 | In vivo/ex vivo Flow cytometry In vitro microscopy |
Oncology Cardiovascular Transplant Rejection Pancreatitis |
Using IVISense Annexin-V 750 for in vivo/ex vivo studies
The generally recommended procedure for in vivo imaging with IVISense Annexin-V 750 is administration via intravenous injection and imaging 2 hours post injection IVISense Annexin-V 750 will clear from tissues after ~3 days after which repeat injection and imaging may be performed for longitudinal studies. However, it is recommended that a pre-injection baseline image be taken prior to reinjection and imaging.
| Route of Injection | Mouse Dose (25 g) | Rat Dose (250 g) | Blood t 1/2 | Tissue t 1/2 | Optimal imaging time | Optimal Re-injection Time (complete clearance) | Route of Metabolism/ background tissue | FMT& IVIS settings |
|---|---|---|---|---|---|---|---|---|
| IV | 100 µL | 300-1000 µg | 5 min | 14 h | 2 h | 3 d | Kidneys (high), liver | FMT 750/770 IVIS 745/800 |
In Vivo Imaging Study
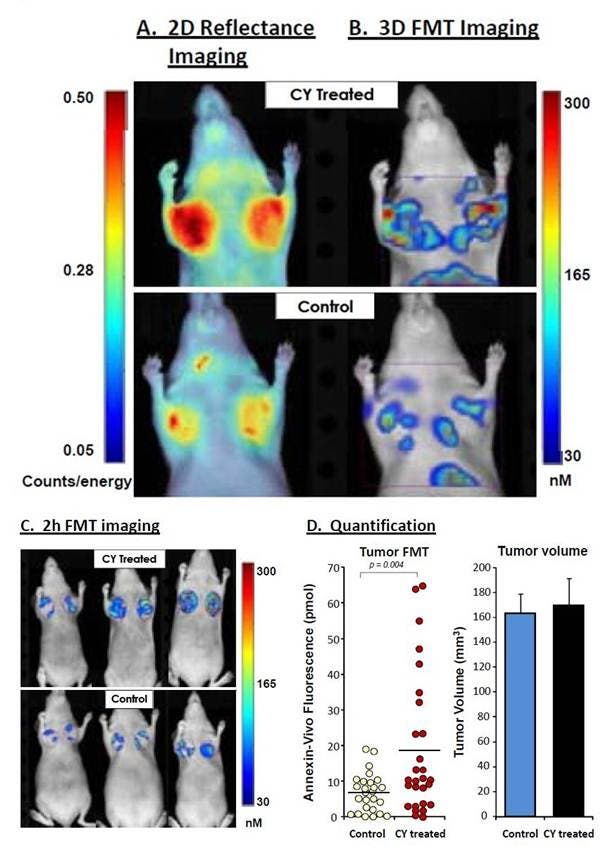
Figure 2: CY-treated and untreated HT-29 tumor xenograft mice were injected with IVISense Annexin-V 750 and imaged two hours later using fluorescence molecular tomography. Figure shows representative images of treated and untreated HT-29 mice selected to reflect the mean group tumor fluorescence intensities. A) The 2D planar fluorescence images are shown. B) The 3D tomographic images show all signal in and around the tumors. C) Representative fluorescence tomography images in HT-29 tumor xenografts are shown from CY-treated mice (upper panels) and control (lower panels), showing only tumor-associated signal. D) Quantification of absolute pmols per tumor in control (n = 26) and CY-treated (n = 28) tumors. The tumor volume data confirms that there was no treatment effect on tumor volumes (as assessed by dimensional caliper measures).
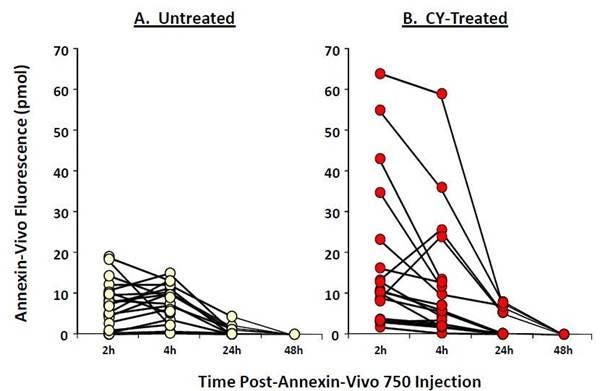
Figure 3: To assess the optimal time-point for imaging IVISense Annexin-V 750 (formerly Annexin-Vivo) in CY-treated tumors, control (A, n=12) and treated (B, n=13) mice bearing HT-29 tumor xenografts were injected with the agent and imaged 2, 4, 24, and 48 hours later.
Ex Vivo Imaging Study
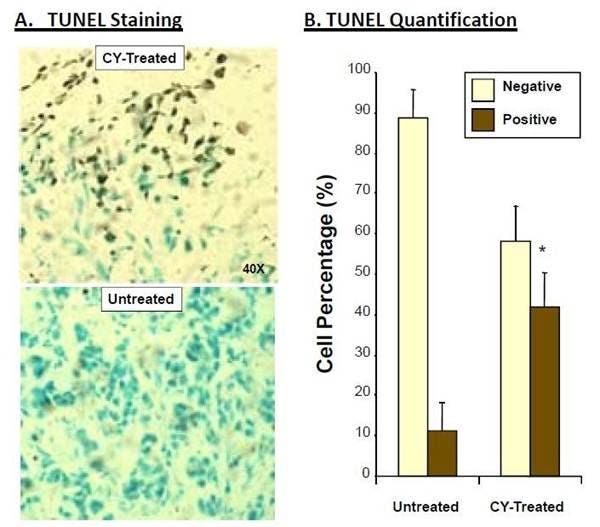
Figure 4: Ex vivo TUNEL staining of tumors from CY-treated and untreated HT-29 tumor xenografts. Tumor tissues were removed from HT-29 tumor xenograft mice, frozen, and sectioned for TUNEL staining. A) 400X microscopy images of TUNEL-stained tissues. B) Cell count results from multiple quadrants of sectioned tissue, represented as staining of total cells.
Flow cytometry and in vitro microscopy
We have validated IVISense Annexin-V 750 for use with fluorescence microscopes and flow cytometers. Here is a brief protocol with a recommended concentration of probe to use:
Resuspend cells in complete medium. Treat with 200 ng/ml anti-FAS antibody for 3-4 h prior to incubation with probe.
Incubate cells with 1 µM IVISense Annexin-V 750 for 10 min at 37 °C.
Wash 1x with PBS.
Flow cytometry filter settings: Add Propidium iodide 1 min prior to acquisition 575/26; 780/60
Fluorescence microscopy filter: Cy5.5 or Cy7
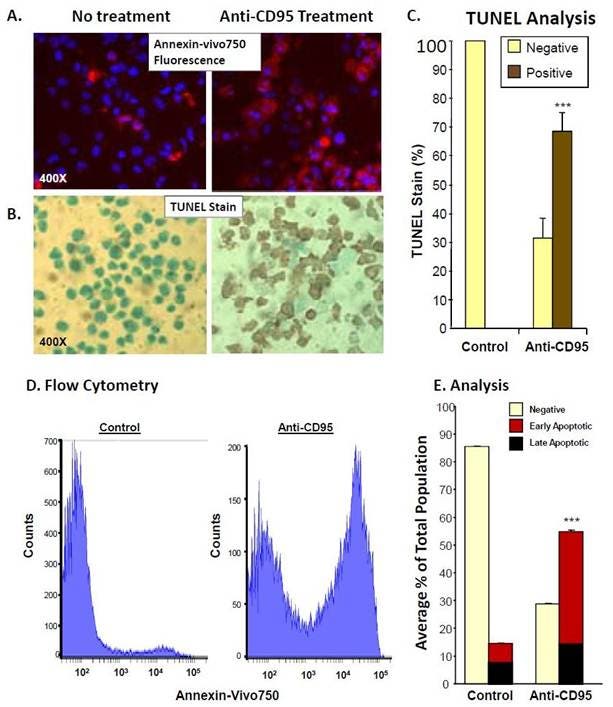
Figure 5: A. IVISense Annexin-V 750 (formerly Annexin-Vivo) was incubated in vitro with treated and untreated Jurkat cells and labeling was compared to TUNEL staining of cells (B). C. Cell counts of TUNEL positive cells by microscopy. D. Jurkat cells induced to apoptosis by anti-CD95 (Anti-FAS) were incubated with IVISense Annexin-V 750 and assessed by flow cytometry. E. Cells in early apoptosis (IVISense Annexin-V 750 positive only) versus late apoptosis. IVISense Annexin-V 750 and propidium iodide double-positive were counted and represented as a percentage of total cells.
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance or use information. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.




























