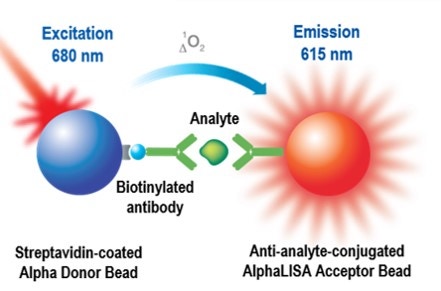
Assay principle

Principle of the AlphaLISA assay technology
AlphaScreen™ and AlphaLISA™ are bead-based assay technologies used to study biomolecular interactions in a microplate format. The acronym "Alpha" stands for amplified luminescent proximity homogeneous assay. As the name implies, some of the key features of these technologies are that they are non-radioactive, homogeneous proximity assays. Binding of molecules captured on the beads leads to an energy transfer from one bead to the other, ultimately producing a luminescent/fluorescent signal. To understand how a signal is produced, one must begin with an understanding of the beads. AlphaScreen and AlphaLISA assays require two bead types: Donor beads and Acceptor beads. Each bead type contains a different proprietary mixture of chemicals, which are key elements of the AlphaScreen technology. Donor beads contain a photosensitizer, phthalocyanine, which converts ambient oxygen to an excited and reactive form of O2, singlet oxygen, upon illumination at 680 nm. Please note that singlet oxygen is not a radical; it is molecular oxygen with a single excited electron. Like other excited molecules, singlet oxygen has a limited lifetime prior to falling back to ground state. Within its 4 µsec half-life, singlet oxygen can diffuse approximately 200 nm in solution. If an Acceptor bead is within that proximity, energy is transferred from the singlet oxygen to thioxene derivatives within the Acceptor bead, subsequently culminating in light production at 520-620 nm (AlphaScreen) or at 615 nm (AlphaLISA). In the absence of an Acceptor bead, singlet oxygen falls to ground state and no signal is produced. This proximity-dependent chemical energy transfer is the basis for AlphaScreen's homogeneous nature.
AlphaLISA and AlphaScreen: understanding the difference
Revvity's Alpha technology includes both AlphaLISA and AlphaScreen assays. Both assays rely on the same Donor beads yet use different Acceptor beads (Figure 2). AlphaScreen Acceptor beads are embedded with three dyes: thioxene, anthracene, and rubrene. Rubrene, the final fluor, emits light detectable between 520-620 nm. In the AlphaLISA Acceptor beads, anthracene, and rubrene are substituted with an Europium chelate. The Europium (Eu) chelate is directly excited by the 340 nm light resulting from the conversion of thioxene to a di-ketone derivative following its reaction with singlet oxygen. The excited Europium chelate generates an intense light detectable within a much narrower wavelength bandwidth centered around 615 nm. In contrast to the AlphaScreen, the AlphaLISA emission is therefore less susceptible to interference by either artificial or natural compounds (such as hemoglobin) that absorb light between 500-600 nm (Figure 3).
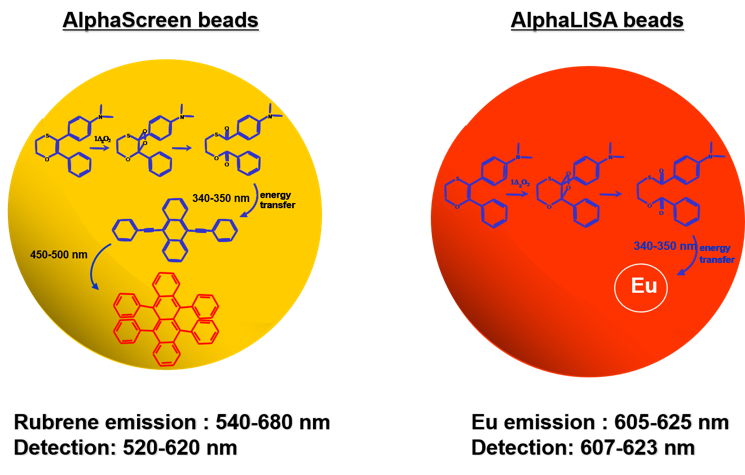
Figure 2. Difference between a TAR (AlphaScreen Acceptor) and a Europium (AlphaLISA Acceptor) bead.
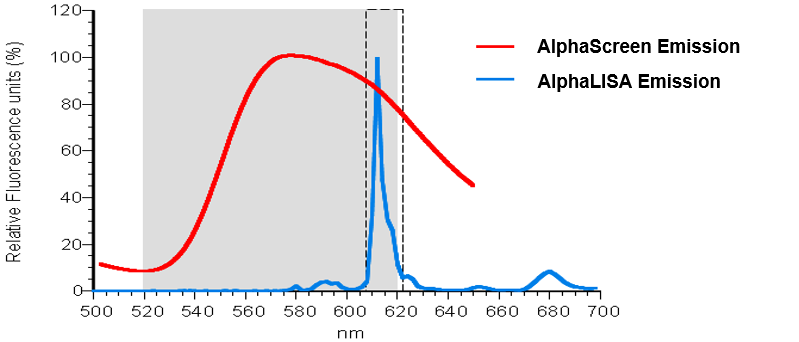
Figure 3. Normalized spectra for AlphaScreen vs. AlphaLISA emission. AlphaLISA Acceptor beads give strong signal, are less prone to matrix interferences (hemoglobin), and are optimized for serum and plasma samples.
Alpha assay features
- Homogeneous assay (no washing steps required)
- Proximity assay with broad energy transfer distance (distance between Donor and Acceptor beads can be up to 200 nm or more, as compared to TR-FRET which has a maximum transfer distance of ~10 nm)
- 96-, 384-, and 1536-well assays
- Biochemical and cell-based assays
- Can be used for low to high affinity binding interactions (pM to mM)
- Compatible with complex matrices such as cell lysates, serum, plasma, CSF, etc. (AlphaLISA)
- Can be used for high-throughput screening (HTS)
Create your own Alpha assay
The information in Create your own Alpha assay will help you design and develop any Alpha assay. The assay format can be an immunoassay using one antibody or a two-antibody sandwich, or it can be a protein-protein interaction assay using a tagged reagent as one or more of the assay components. Many other assay variations are also possible to develop.
Applications
- AlphaLISA immunoassay kits
- Alpha SureFire® no-wash cellular kinase assay kits
- Immunoassays
- Protein-protein and protein-nucleic acid interactions
- Ligand:receptor assays
- Kinase assays
- Epigenetics & post-translational modifications
- Protease assays
- Immunogenicity assays
- Antibody detection and characterization assays
- cAMP assays
- EMSA conversion assays
- Other Alpha applications
- Working with serum and other biological matrices in an AlphaLISA assay
- Working with cell extracts and supernatants
Alpha Citations
View a listing of selected Alpha citations, grouped by application.
Instrument Options & Settings
Alpha technologies require excitation at 680 nm and reading emission at 520-620 nm (for AlphaScreen) or at 615 nm (for AlphaLISA). A laser excitation source (rather than a Xenon Flashlamp) is usually needed, as an intense light source is required to generate signal. Many basic fluorescence plate readers are not equipped to run Alpha assays. We recommend using our EnVision® (with Alpha module), EnSight™, or VICTOR™ Nivo™ Multilabel Plate Reader, each of which comes with pre-set Alpha settings. You can use the same generic instrument protocol for AlphaScreen and AlphaLISA assays. If you are using a plate reader from another company, we recommend that you check to see if the manufacturer has validated the instrument for an AlphaScreen or AlphaLISA assay. This information is usually found on the company's website. Revvity has not validated other instruments, and we would recommend that you speak to the instrument manufacturer for information on the recommended settings to use. If you are using an EnVision Multilabel Plate Reader, you may notice there are two instrument protocols for Alpha. One is the HTS Alpha protocol, and other is the standard Alpha protocol. The HTS Alpha protocol is to be used when you are performing high-throughput assays, and when the time it takes to read each plate is an important factor for you.
Microplate Selection for AlphaLISA
| Microplate | Catalog number | Color | Assay volume | Comments |
|---|---|---|---|---|
| ½ AreaPlate-96 | 6002290 | white | 50 µL | Half area plates are 96-well format plates with lower reaction volumes than standard 96-well plates, and are recommended for most AlphaLISA biomarker kits. |
| ½ Area AlphaPlate | 6002350 | light gray | 50 µL | Gray version for decreased cross-talk for low volume assays in 96-well format. |
| OptiPlate™-96 | 6005290 | white | 100 µL | |
| CulturPlate™-96 | 6005680 | white | 100 µL | Coated for use in tissue culture |
| OptiPlate-384 | 6007290 | white | 50 µL | The 384-well plate that is recommended for the highest sensitivity in a 50 µL reaction |
| AlphaPlate®-384 | 6005350 | light-gray | 50 µL | Light gray color reduces potential for well-to-well crosstalk |
| ProxiPlate™-384 Plus | 6008280 | white | 20 µL | |
| AlphaPlate-1536 | 6004350 | light-gray | 10 µL | Light gray color reduces potential for well-to-well crosstalk |
Tips & FAQs
Tips
- Some Alpha beads are light-sensitive. Where possible, perform Donor bead dilution and addition away from direct sunlight or with bright overhead lights turned off (<100 lux, similar to hallway lighting). The lighting should still be bright enough to allow accurate pipetting.
- The Alpha signal is temperature-dependent. Make sure plates are near 22°C when being read. If you are running the assay on different days, you will want to keep incubation temperatures and plate-read temperatures consistent.
- If you are using our clear TopSeal™-A plate seal, you have the option to leave it on your plate when reading the Alpha signal
FAQs
Assay Applications
Q. What is the main difference between AlphaLISA and AlphaScreen assays?
A. Both assay formats use the same type of Donor bead. The main difference between the two assays is in the Acceptor beads. With AlphaScreen Acceptor beads, the final light emission is from rubrene; with AlphaLISA Acceptor beads, the final light emission is from Europium. The emission wavelength is different between AlphaScreen beads (520-620 nm) and AlphaLISA beads (615 nm). Because of the narrow emission spectrum of AlphaLISA beads, there is much less interference in serum and plasma samples and better sensitivity is obtained. Note: When switching between AlphaScreen and AlphaLISA assays, there is no need to change the filter in the EnVision or the EnSpire Multilabel Plate Reader.
Q. What are Omnibeads used for?
A. Omnibeads are typically used as instrument controls. Each Omnibead contains both the chemicals in an Alpha Donor bead and the chemicals in an AlphaScreen Acceptor bead.
Q. What are TruHits beads used for?
A. TruHits beads are typically used as assay controls for Alpha assays. They contain streptavidin-coated Donor beads and biotin-coated AlphaScreen or AlphaLISA Acceptor beads, which should be able to interact on their own in the absence of other reagents. They can be used to determine whether a molecule or matrix interferes with an AlphaScreen assay, or to screen small molecule hits for false positives.
Q. How do Alpha assays compare to TR-FRET assays?
A. The AlphaScreen and AlphaLISA assay platform is much more flexible than TR-FRET. The AlphaScreen and AlphaLISA assay platform can allow for detection of analytes ranging in size from small molecules to large viral particles, with a maximum distance of 200 nm between the Donor and Acceptor beads. TR-FRET based detection systems do not have that flexibility. In addition, AlphaLISA assays can be run in serum or culture media, which can be problematic for TR-FRET assays.
Q. Can I use AlphaLISA assays for diagnostic testing?
A. Absolutely not. AlphaLISA kits are developed for research use only. Not for use in diagnostic procedures.
Q. Can the Alpha assay be automated for HTS?
A. Yes. No wash steps are required and low sample volumes can be used, so it is very simple to miniaturize and automate the AlphaLISA assay using the JANUS® Automated Workstation from Revvity.
Assay Protocols and Options
Q. How light sensitive are Alpha assays?
A. Streptavidin-coated Alpha Donor beads are somewhat light sensitive. AlphaLISA Acceptor beads are not light-sensitive. Direct sunlight and intense artificial light can activate Donor beads, releasing singlet oxygen that can react with streptavidin bound to the Donor-bead and decrease its efficiency. Inadvertent exposure to excess light levels can reduce signal strength but Im most cases the assay will still give good results. Ideally, steps involving Donor beads should be done under subdued laboratory light levels of less than 100 Lux (see table below).
Alternatively, green filters (LEE 090 filters (preferred) or Roscolux filters #389 from Rosco) can be applied to light fixtures. Incubations of Alpha assays should be performed in the dark, with the microplate covered by our black TopSeal (Cat. No. 6050173), a second opaque microplate or lid, or sheet of foil.
What does 100 lux look like?
| Lux level | Environment |
|---|---|
| 320-500 Lux | Office lighting |
| 100 lux | Dark overcast day |
| 80 lux | Hallway lighting |
| 50 lux | Family living room |
Q. What is the difference between the various AlphaLISA buffers?
A. AlphaLISA Immunoassay buffer is used in most of the AlphaLISA kits in the market. In cases where high background is a concern, AlphaLISA HiBlock buffer is provided in the kit. The HiBlock buffer is also available separately. The AlphaLISA Immunoassay buffer is not a lysis buffer per se, but it does contain detergent and low salt, so it can be used to lyse cells in some assays. For membrane proteins, the AlphaLISA immunoassay buffer has been shown to be sufficient to expose the protein epitopes for AlphaLISA detection. Our AlphaLISA Lysis buffer (catalog number AL003) was developed specifically for use in cell-based assays. This buffer contains SDS in order to lyse the cells and solubilize the proteins. This buffer is required when detecting intracellular proteins. AlphaLISA Universal buffer is the buffer used to QC all AlphaLISA and AlphaScreen Toolbox beads. It is universal since all QC assays will work in it, but it may not be the optimal buffer for all AlphaLISA assays.
Q. Do I need to shake the plate before I read? How big are the Alpha beads and do they settle?
A. Alpha beads are small (250-350 nm diameter). They will not settle or clog pipet tips, and thus do not require shaking before a read.
Q. What instruments can read an Alpha assay? Can my luminometer do it?
A. Alpha assays require a dedicated laser at 680 nm for excitation of the reaction. Xenon Flash based systems and luminometers will not work for Alpha assays. The Revvity Envision and EnSpire instruments are specifically designed to read Alpha assays. The VICTOR™ reader cannot be used because it does not have a built-in laser. While some instruments from other suppliers claim to be compatible with Alpha assays, the assays have been optimized for use with the Revvity instruments.
Q. Can I conjugate my own antibody or use a fusion tag to bind my protein to the Acceptor beads?
A. Yes, we have optimized protocols, unconjugated beads, and many types of Donor and Acceptor beads coupled to secondary antibodies, or to glutathione or nickel chelate for capture of expression tags. We also provide a custom conjugation service. Please refer to Create your own Alpha assay for information on bead conjugation, and to Alpha products and catalog numbers for a listing of available catalog products.
Q. Why is the Immunoassay buffer so "bubbly"?
A. Our Immunoassay buffer is adapted for better detection of analytes in high protein concentration samples, such as serum or cell lysates. It is engineered for optimal immunocomplex formation while minimizing background. If dispensing is causing excessive bubbling, anti-foaming agents are available. Alternatively, a detergent-free buffer could be tested.
Q. How long are Alpha Donor and Acceptor beads stable once they are diluted in buffer?
A. Long term stability of diluted beads has not been evaluated. However, diluted beads will be stable for more than 12 hours, allowing automated screening and other HTS assays.
Q. Can changes in temperature influence the signal?
A. Yes. AlphaLISA and AlphaScreen assays are temperature sensitive. The incubation temperature can influence the rate at which the interaction between antibodies and analyte approaches equilibrium. The temperature of the plate reader can affect the rate of generation and diffusion of singlet oxygen. Changes as high as 10% per °C can be observed, depending on the assay. For the most uniform results, try to keep the temperature of the final microplate incubation as close possible to that of the plate reader.
Effects of Sample Components
Q. What buffers are incompatible with Alpha assays? Does it matter what buffer I use?
A. Commonly used buffers such as HEPES, PBS, and Tris are compatible with AlphaScreen. Various buffers may affect the performance of a detection assay.
Q. Can RPMI media be used for AlphaLISA and AlphaScreen assays?
A. RPMI medium can be used for AlphaLISA assays. However, due to the presence of a high level of free biotin, a decrease in total counts and in signal:background ratio can be expected. Also, the presence of free biotin might affect the LDL (lower detection limit). In most cases, satisfactory results can still be obtained using RPMI medium. Increasing the concentration or the incubation time of the streptavidin-coated Donor beads can help to overcome the presence of biotin in RPMI media in some cases. Other cell culture media that work well in Alpha assays include DMEM and MEM.
Q. Do DMSO, SDS, EDTA, or sodium azide interfere with Alpha assays?
A. AlphaScreen and AlphaLISA assays can tolerate up to 10% DMSO, 0.02% SDS, 100 mM EDTA, and 0.005% sodium azide. Above these concentrations the signal will decrease, but acceptable results often can be obtained. Note that other components of your assay might not tolerate such concentrations (e.g. analyte & protein).
Q. Does phenol red interfere with the AlphaLISA and AlphaScreen assay?
A. Phenol red does not interfere with the AlphaLISA assay signal, but will interfere in an AlphaScreen assay.
Q. What compounds in my library could interfere with the AlphaScreen or AlphaLISA assay?
A. Not all libraries are created equal. The main mechanisms of compound interference with the Alpha assay signal are by: 1) quenching the singlet oxygen, 2) competing against the interaction between biotin and streptavidin, and 3) acting as an inner filter. Strong singlet oxygen quenchers are transition metals (Fe2+, Fe3+, Cu2+, Ni2+, Zn2+, Al2+) when used in the µM range, anti-oxydants such as azide and ascorbate, compounds with structures based on thiophene (100 µM to 10 mM), and heme-containing transition metals (such as hemoglobin). Competitors of the interaction between biotin and streptavidin are biotin-like structures, while inner filters are compounds that will absorb light at 520-620 nm (emission) and/or at 680 +/-5 nm (excitation). Thus, blue/green compounds will act as inner filters. You can use our TruHits beads as a control, to test whether a compound is interfering in AlphaScreen format.
Troubleshooting Tables
Our Alpha troubleshooting tables contain general troubleshooting information and detailed troubleshooting for AlphaLISA assays.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























