Overview
AlphaLISA™ assays are homogeneous, no-wash immunoassays with high analytical sensitivity and wide dynamic ranges. Compared to standard ELISA protocols, AlphaLISA assays increase throughput while substantially decreasing hands-on and total assay times. AlphaLISA assays are versatile and can be used to detect analytes that are secreted, intracellular, or membrane-bound. In addition to providing high-quality data and robust performance, AlphaLISA assays are simple and quick to optimize. They are able to be miniaturized and automated for increased laboratory productivity. The AlphaLISA platform is, thus, an excellent fit for the immunodetection of biomarkers and is now emerging as the new-generation immunoassay technology in drug discovery, preclinical studies, and basic research.

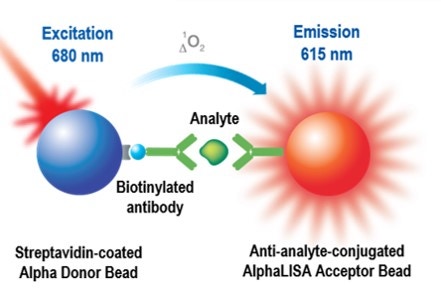
Fig. 1. Detection of analyte in AlphaLISA format, using a sandwiching antibody assay
Each AlphaLISA immunoassay kit has five components:
- AlphaLISA Acceptor beads coated with anti-analyte antibody #1
- Streptavidin-coated Donor beads
- Biotinylated anti-analyte antibody #2
- Lyophilized analyte
- A 10X AlphaLISA buffer
The kits can be used in a 96-, 384-, or 1536-well format.
What do I need to run this assay?
Required reagents available from Revvity:
- AlphaLISA kit
- Microplates (We recommend our 96-well 1/2 AreaPlates or our 384-well white OptiPlates™). Also see Microplate selection.
- TopSeal™-A adhesive plate seal for incubations
Other reagents:
- Your experimental samples
- Lysis buffer, if applicable (see more information on Working with cell extracts and supernatants in Alpha assays)
Instrumentation/Equipment:
- A plate reader (capable of reading Alpha assays). See more information on instrumentation on the Alpha technology main page.
Protocols
All protocols can be found on the product tech data sheets. In general, the basic protocol involves first mixing your sample or standards with one bead/antibodies, incubating, then adding the second bead, incubating, and reading.
1-step, 2-step, and 3-step assay protocols
- 1-step assay: mixing both antibodies, sample, and beads together all in one step
- 2-step assay: mixing the antibodies, sample, and Acceptor bead together first, incubating, then adding Donor bead for final incubation
- 3-step assay: mixing sample first with one of your antibodies, incubating, adding the second antibody, incubating, then adding Donor bead for final incubation
Each AlphaLISA kit was developed as either a 2- or 3-step assay. The order of addition can have a dramatic effect on assay analytical sensitivity. We recommend that the first time you run the assay, you use the recommended protocol for the kit you are working with. To streamline your experiment, you can later develop the assay as a 1-step protocol. Keep in mind, however, that assay analytical sensitivity may decrease. Conversely, you can change a 2-step assay to a 3-step protocol; this may increase the analytical sensitivity of your assay for some kits/sample matrices. This will need to be tested on a case-by-case basis.
Using increased sample volume to increase analytical sensitivity
All AlphaLISA immunoassay kits have protocols that call for 5 µL of sample in a 50 µL reaction volume. For some kits and some matrices, increasing the proportion of the sample in the assay can increase analytical sensitivity. If the analytical sensitivity of your assay is too low, you can try adding up to 20 µL of sample in a 50 µL reaction. You will need to re-adjust the dilutions of Donor beads, Acceptor beads, etc. so that the final concentration of antibodies and beads is the same as in the original protocol. Also, you will need to adjust your standards so that you are adding 20 µL of standard to create your standard curve. View our protocol alteration for the AlphaLISA TNFalpha kit.
Please note that increasing the volume of sample in a 50 µL reaction might also increase any potential assay interference arising from your sample or matrix. We do not recommend adjusting the volume of your sample if you are using serum samples.
Preparing analyte-depleted serum
We recommend that you check the tech data sheet for your kit to determine the ideal matrix; many of our kits have already been validated to work with serum samples. When analyzing samples in serum, it is best to prepare the standard curve in a sample matrix as similar as possible to the actual samples. This will help ensure that the assay accuracy is as high as possible. Analyte-depleted serum can be prepared using our protocol, or you may be able to purchase it from a supplier such as Bioreclamation.
Data processing
More information on processing AlphaLISA immunoassay data, including various methods of data processing, sample data, and step-by-step instructions using GraphPad Prism®.
Citations
Brief listing of citations for Alpha biomarker detection assays.
To search our database of citations referencing Revvity products, please visit the Revvity Life Sciences Citations Library.
Tips and FAQs
View our AlphaLISA kit references tables for information on species selectivity, lower and upper limits of detection, dynamic range, and tested matrices.
- Most AlphaLISA assays can be performed on samples in buffer, serum, or cell culture medium. Many users also perform these assays in plasma. Check the matrix compatibility and species cross-reactivity tables for your specific assay.
- AlphaLISA assays should be run in white microplates to maximize signal. To select the best microplate for your assay volume, see our AlphaLISA microplate selection table.
- Set up the standard curve using analyte diluted in the same matrix as the samples (serum, culture medium, etc.).
- AlphaLISA Donor beads are somewhat light-sensitive. Where possible, perform Donor bead dilution and addition away from direct sunlight or with bright overhead lights turned off (<100 lux). The lighting should still be bright enough to allow accurate pipetting.
Q. Can I create my own AlphaLISA kit?
A. Yes. In addition to custom assay development and custom bead conjugation, we offer unconjugated beads and information on how to Create your own Alpha assay.
Q. The analyte that I want to measure is not on your website. Do you have it?
A. We only have the indicated analytes at present, but your feedback is very important to us. New analytes are in development, so please contact Revvity with your specific request. If your analyte of interest is not listed, we can develop a custom assay to your specifications. You can also purchase an AlphaLISA Toolbox and develop your own assay using our many types of Donor and Acceptor beads.
Q. Can I use AlphaLISA assays for diagnostic testing?
A. Absolutely not. AlphaLISA kits are developed for research use only. Not for use in diagnostic procedures.
Q. How many assay points can be done with an AlphaLISA kit?
A. The kits exist in two formats: 500 assay points and 5,000 assay points, based on an assay volume of 50 µl in 96- or 384-well plates using the kit components at the recommended concentrations. By reducing assay volume while maintaining component concentrations, more assay points can easily be performed.
Q. How many AlphaLISA assay points can be generated in a 1536-well assay format?
A. Alpha assays can be miniaturized from 50 µl to 10 µl for use in a 1536-well microplate by using lower reagent volumes and identical final reagent concentrations. As a result, the number of assay points will be increased 5 times, allowing the generation of 2,500 assay points with the small kit and 25,000 with the large kit.
Q. Do I need to shake the plate before I read? How big are the Alpha beads and do they settle?
A. Alpha beads are small (250--350 nm diameter). They will not settle or clog pipet tips, and thus do not require shaking before a read.
Q. How do Alpha assays compare to TR-FRET assays?
A. The AlphaScreen™ and AlphaLISA assay platform is much more flexible than TR-FRET. The AlphaScreen and AlphaLISA assay platform can allow for detection of analytes ranging in size from small molecules to large viral particles, with a maximum distance of 200 nm between the Donor and Acceptor beads. TR-FRET based detection systems do not have that flexibility. In addition, AlphaLISA assays can be run in serum or culture media, which can be problematic for TR-FRET assays.
Q. Why is the ImmunoAssay buffer so “bubbly”?
A. Our ImmunoAssay buffer is adapted for better detection of analytes in high protein concentration samples such as serum or cell lysates. It is designed for optimal immunocomplex formation while minimizing background. If dispensing is causing excessive bubbling, anti-foaming agents are available. Alternatively, a detergent-free buffer could be tested.
Q. Can I use AlphaScreen and AlphaLISA assays with whole blood samples?
A. The overall assay signal can be expected to decrease when blood or hemoglobin is present in the assay, but the signal-to-background ratio (S:B) and lower detection limit (LDL) may still be acceptable. The effects of blood or hemoglobin in your samples should be determined for each AlphaLISA assay.
Q. How much sample volume can be used for an AlphaLISA assay?
A. The standard sample volume is 5 µl, but the reactions performed in buffer can be adapted to allow addition of up to 20 µl of sample to increase analytical sensitivity. For reactions performed in sample matrices other buffer, the maximum sample volume should be determined for each analyte.
Q. Can I improve the analytical sensitivity of the AlphaLISA kit?
A. There are four ways to try to improve the analytical sensitivity of a kit. The first way is to increase the volume of sample you add to a 50 µl reaction. See an example of how to do this in the Protocols section. The second way is to change a 2-step assay into a three-step assay. Instead of mixing your sample with the biotinylated antibody and the Acceptor bead at one time, you will be mixing the sample first with the Acceptor bead, incubating, then adding the biotinylated antibody for a second incubation before adding Donor bead. The third way is to lengthen the protocol incubation times, to allow more time for antibody binding to the analyte in your sample. The fourth way is to use a high concentration protocol. This can be effective if you are working in a complex matrix, such as serum:
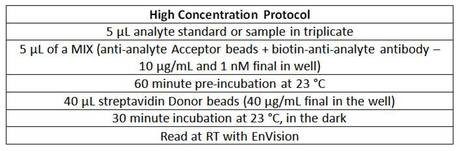
High concentration protocol
Q. How should I prepare my standard curve?
A. Refer to your kit manual for detailed information on what volumes to use. Make sure you use the same matrix your samples are in. For example, if your samples are in human sera, we recommend you create your standard serial dilution in analyte-depleted human serum or FBS (depending on which kit you are using). Refer to the tech data sheet or manual for your particular kit to find the recommended matrix if you are working with serum samples.
Q. Can I get extra vials of standard?
A. Yes. Please refer to the Alpha products and catalog numbers page for a listing of catalog numbers.
Q. I'm running serum samples. How can I buy or prepare analyte-free serum for my standard curve?
A. Revvity does not supply analyte-free serum, but you may be able to purchase it from a supplier such as Bioreclamation. Alternatively, click here for a protocol you can use for this purpose.
Q. What is the shelf life of each kit?
A. The kits should be stable for at least 12 months from the manufacturing date when stored in their original packaging at the recommended storage conditions.
Q. What is the stability of the Alpha assay standard after dilution?
A. The aliquoted standard is stable for 45 days at -20ºC. The stability at 4ºC will depend on each analyte.
Q. Does phenol red interfere with the AlphaLISA assay?
A. Phenol red does not interfere with the AlphaLISA assay signal.
Q. Can RPMI media be used for AlphaLISA assays?
A. RPMI medium can be used. However, due to the presence of a high level of free biotin, a decrease in total counts and in signal-to-background ratio (S:B) can be expected. Also, the presence of free biotin might affect the LDL (lower detection limit). In most cases, satisfactory results can still be obtained using RPMI medium. Other cell culture media that work well in Alpha assays include DMEM and MEM.
Q. Can the AlphaLISA assay be automated for HTS?
A. Yes. No wash steps are required and low sample volumes can be used, so it is very simple to miniaturize and automate the AlphaLISA assay using the JANUS™ Automated Workstation from Revvity.
Q. Do DMSO, SDS, EDTA or sodium azide interfere with AlphaLISA assays?
A. AlphaLISA assays can tolerate up to 10% DMSO, 0.02% SDS, 100 mM EDTA, and 0.005% sodium azide. Above these concentrations the signal will decrease, but acceptable results often can be obtained. Note that other components of your assay (e.g. analyte & protein) might not tolerate such concentrations.
Q. Revvity offers an AlphaLISA P24 kit and an ELISA P24 kit. Which should I use?
A. The best choice of assay depends on your assay requirements:
| Requirement | Recommended Assay |
|---|---|
| Short protocol | AlphaLISA |
| Analytical Sensitivity | ELISA |
| Dynamic range | AlphaLISA |
| No wash step | AlphaLISA |
| Small sample volume | AlphaLISA |
| Sample matrix: cell culture | AlphaLISA |
| Sample matrix: cell culture, serum & plasma | ELISA |
| High throughput | AlphaLISA |
| Instrumentation |
ELISA (any photometric reader) |
| AlphaLISA (requires Alpha reader) |
Q. What is the difference between the AlphaLISA ImmunoAssay buffer, HiBlock buffer, AlphaLISA Lysis buffer, AlphaLISA Universal buffer, AlphaLISA NaCl buffer, AlphaLISA Dissociation buffer, and AlphaLISA Epi buffer?
A. AlphaLISA ImmunoAssay buffer is used in most of the AlphaLISA kits in the market. In cases where high background is a concern or where the assay has special requirements, other buffers are included in the kit. More details...
Q. Why does the Alpha assay titration assay curve have a bell shape?
A. The titration curve has a bell shape because of the "hook effect". Once the beads are saturated (maximum binding), excess analyte will inhibit Acceptor and Donor bead association and cause a decline in signal.
Q. I am measuring a biomarker in a cellular model. Can I perform the AlphaLISA assay directly in a cell culture plate and avoid the transfer step?
A. Yes. AlphaLISA assays can generally be performed in the presence of cell culture media and cells. Minor optimization might be needed depending on your cellular model.
Q. How much total volume of serum can be used in the assay?
A. Due to the fact that the hemoglobin in the serum will capture some of the oxygen released by the Donor beads, the percentage of serum should not exceed 10% of the total volume (i.e. 5 µl serum sample in 50 µl final assay volume).
Q. How do I calculate the lower detection limit (LDL) of an AlphaLISA assay?
A. Test the blank 12 times within the same run. The LDL is mean ± 3 standard deviations when read on the standard curve.
Q. How can I decrease analytical sensitivity of my Alpha assay?
A. You can decrease the analytical sensitivity by adding less sample (i.e. 2 µL instead of 5 µL). Changing the incubation time might have an effect as well, but this is assay-specific. You could also change from a 2-step protocol to a 1-step protocol.
Q. Why do I need to change tips when diluting and loading reagents?
A. If the tips are not changed at each serial dilution step, a false value will be obtained for the LDL. Tips MUST be changed while diluting the analyte. It is not necessary to change tips when loading the microplate if the reagents are always added starting with the lowest concentration and moving toward the highest concentration.
Troubleshooting
View our Alpha troubleshooting tables.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























