Overview
Alpha technologies can be used to study biomolecular interactions, including protein-protein, protein-peptide, protein-nucleic acid, protein-small molecule, and carbohydrate-lectin interactions, as well as nuclear receptor binding. Our Alpha technology can also be used to study the interaction of a protein with a library of other entities. Assays can be biochemical in nature (using purified reagents). The use of our AlphaLISA™ beads allows you to study events in complex matrices, such as cell lysates, supernatants, plasma, and serum. The availability of many different standard catalog "toolbox" bead products provides assay flexibility.
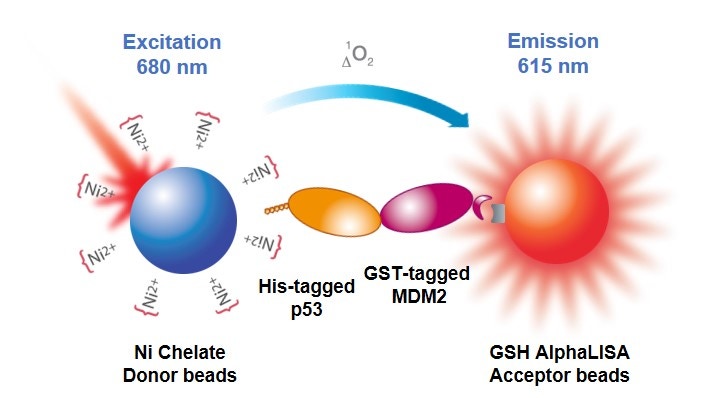
In an Alpha biomolecular interaction assay (Figure 1), beads are selected, and the biomolecules of interest are tagged appropriately, so that one biomolecule associates with the Donor bead and the other biomolecule associates with an AlphaLISA or AlphaScreen™ Acceptor bead. If the two biomolecules interact, the Donor and Acceptor beads are brought together, and excitation of the Donor beads results in generation of signal. You could also work with antibodies against your biomolecules if they are not tagged or use a biotinylated biomolecule associated with a streptavidin-coated bead. If you are studying a weaker interaction, you may want to consider using Ni-NTA and glutathione beads, if possible, for reasons described further below.

Figure 1. Protein-protein interaction assay in Alpha format, using His-tagged p53, GST-tagged MDM2, glutathione AlphaLISA Acceptor beads, and nickel chelate Alpha Donor beads.
What do I need to run this assay?
Required reagents available from Revvity:
- Alpha Donor bead
- AlphaScreen or AlphaLISA Acceptor bead
- Microplates – We recommend our 96-well 1/2 AreaPlates or our 384-well white OptiPlates™. Also see Microplate selection.
- TopSeal™-A adhesive plate seal for incubations
Instrumentation/equipment:
- A plate reader capable of reading Alpha assays (We recommend the Revvity EnVision™ or EnSight™ Plate Reader.)
- Optional: plate rocker
Assay considerations
Buffer selection
Visit Buffer selection for Alpha assays for help in choosing a suitable buffer for your assay. This page describes the various Alpha immunoassay buffers supplied by Revvity and will help you avoid interfering substances in buffers of your own design.
Assay design
In addition to the information below, we recommend you look at the information in our Create your own Alpha assay section for help with assay development.
Equilibrium
For a biomolecular interaction assay, you will need a certain amount of AB interaction for signal (Figure 2).
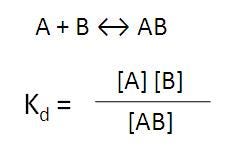
Figure 2. Equilibrium of binding of molecule A to molecule B.
If the interaction between A and B is strong (as in an antibody-analyte interaction), there will be lots of AB complex. If the interaction between A and B is weaker, as in most protein-protein interactions or a glutathione-GST or Ni-NTA-His tag interactions, you may need to increase concentration of one protein or the other to drive equilibrium towards more AB complex, or increase the amount of both to generate more AB complex. Binding affinities for various interactions are shown in Figure 3.
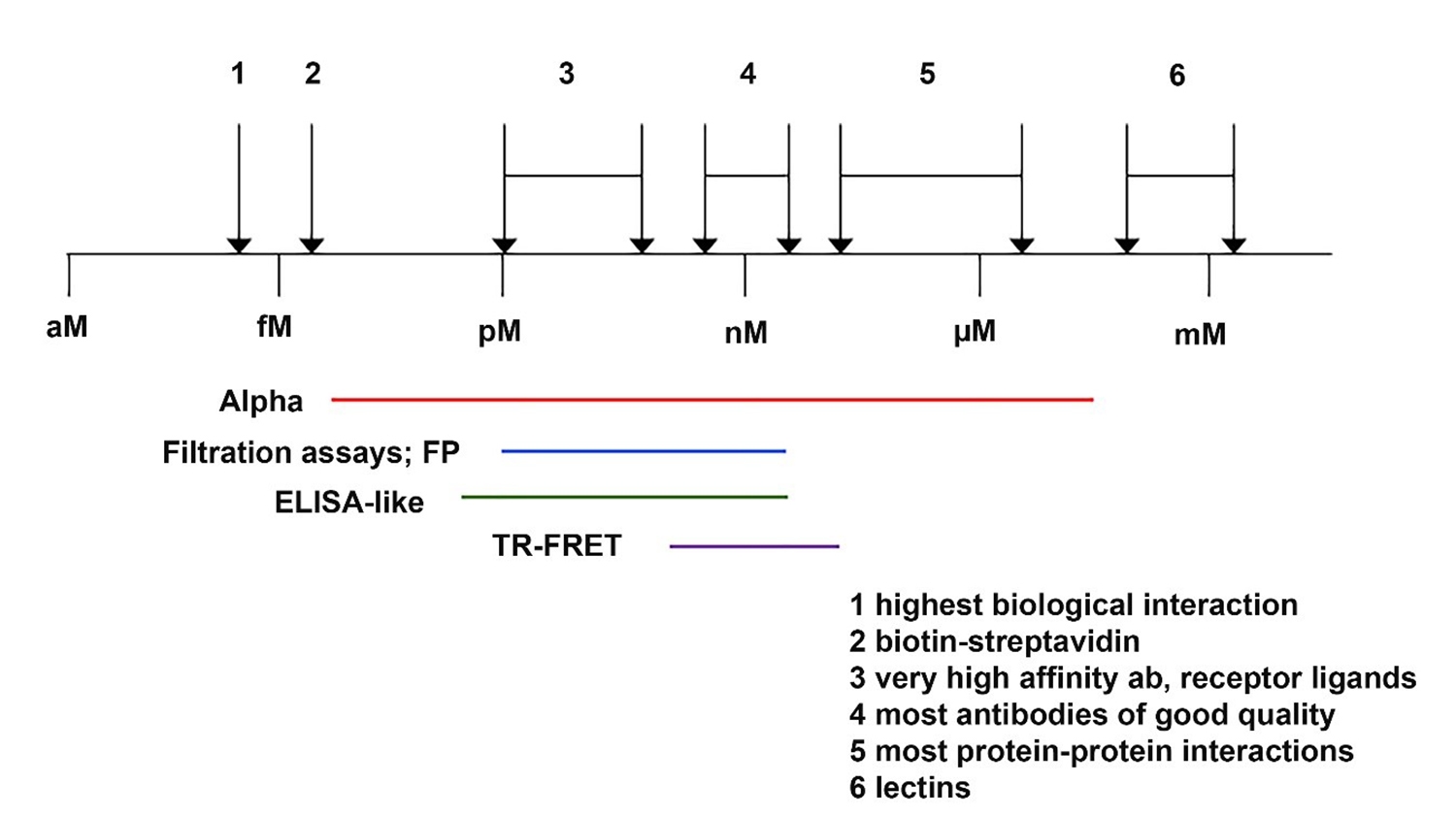
Figure 3. Binding affinities for various interactions.
Keep in mind what the goal of your assay is. Are you looking for something that will either increase or break the protein-protein interaction? Will you be using antibodies to try to block the interaction? If so, you probably don't want to use Protein A beads in your assay design.
Tags
A. Fc-tags
When using antibodies to associate entity with a bead, check to see if it is a “blocking” or “non-blocking” antibody. Blocking antibodies will usually block epitope recognition.
B. Non-peptidic
Digoxigenin (DIG), biotin, and fluorescein/FITC are all tags that can be used with our beads. These tags are usually added exogenously to proteins and peptides, via chemical coupling reactions. Revvity provides protein and peptide biotinylation and FITC labeling custom services. These tags can also be incorporated into nucleic acids by using a labeled nucleotide analog during oligo synthesis or via end-labeling reactions. Other companies provide oligo custom synthesis with DIG, biotin, and fluorescein/FITC modifications. Depending on your labeling method, it can be hard to control the number of tags per molecule. You should consider if your oligos or peptides need to be end-labeled for your assay. If you find that you need to biotinylate a protein, there are essentially two approaches for adding biotin to a full-length protein. The first method is by chemical reaction (for example, biotinylation using SoluLink NHS-biotin). The second method is by engineering a recombinant form of the protein encoding a biotinylation sequence that may be labeled in vivo or in vitro (for example, using the Biotin AviTag™ system from Avidity, LLC). The latter choice offers a more-specific labeling of the binding partner.
C. Peptidic
Peptidic tags, such as 6xHis, GST, c-Myc, FLAG, and HA can all be used with our beads. These tags are usually encoded into the recombinant expression plasmid or during peptide synthesis. These tags vary in length, from several amino acids to 100+ residue proteins. You can position the tag at the C-terminus, N-terminus, or within the amino acid sequence of your protein as desired to prevent steric hindrance of your binding interactions.
Bead Capacity
There is a limited capacity on the bead. You can try to push the equilibrium of the binding reaction by adding more of one partner or the other, but you need to be careful not to exceed the capacity of the system (leading to a hook effect).
As an example, the amount of glutathione on the surface of a bead is more than the amount of anti-GST antibody on the surface of beads. But you still would have to consider how much GST- tagged protein could interact with either bead. Because the interaction of the GST tag with glutathione is weaker, you can add more GST-tagged biomolecule before saturating the glutathione bead. Because the interaction of the GST tag with an anti-GST antibody is stronger, you would reach the hook point more easily than with a glutathione bead.
- The theoretical maximum capacity of a streptavidin-coated bead at 20 µg/mL of bead is 30 nM. Please note that this does not take into consideration the size of the biotinylated molecule that will associate with the bead. For example, you may find that a biotinylated antibody will saturate the bead at 2-3 nM, rather than at 30 nM. If you have saturated the bead, you may see a hook effect.
- The theoretical maximum capacity of an antibody-coated bead is 3-10 nM. Please note this is a theoretical number – you may be able to add more or less than 3-10 nM "antigen" to your assay before hooking, depending on how strong the antibody-antigen interaction is.
- The theoretical maximum capacity of a Ni-NTA or glutathione bead for a tagged protein is ~ 30 nM. However, because the His-Ni-NTA and GST-glutathione interactions are weak compared to other interactions (and more tagged protein will be dissociated from the bead at equilibrium), you will likely be able to use 300-1000 nM tagged protein before reaching the hook point.
Affinity
Here are some examples:
A. For streptavidin-coated bead + biotinylated component
- Pros: The binding between streptavidin and biotinylated component is very strong, so most of the biotinylated component will be associated with the bead, rather than being free in solution.
- Cons: You can exceed the bead binding capacity more quickly because the binding is so strong. So, if you try to add more biotinylated reagent to push the equilibrium to AB complex, there is a higher risk of hooking.
B. For antibody/protein A bead + protein:
- Pros: The binding between antibody and protein is fairly strong, so most of the antibody will associate with your protein.
- Cons: You can exceed the binding capacity more quickly because the binding is fairly strong. So, if you need to push equilibrium by adding higher concentration of protein, there is a higher risk of hooking at the higher concentration.
C. For anti-GST bead + GST-tagged protein:
- Pros: The binding between antibody and tagged protein is fairly strong, so most of the antibody will associate with your tagged protein.
- Cons: You can exceed the binding capacity more quickly because the binding is fairly strong.
D. For Glutathione bead + GST-tagged protein:
- Pros: This affinity is weaker, so you won’t exceed the binding capacity as quickly; you can add more GST-tagged protein if you need to push a binding equilibrium to AB complex.
- Cons: The affinity is weaker, so there will be a larger proportion of GST-tagged molecules that is associated with the bead.
In most cases, because protein-protein interactions tend to be weaker, it may be better to use Ni-NTA bead, GSH beads, etc. as opposed to using streptavidin beads or antibody-associated beads.
Titration of each protein in an Alpha assay
From 0 to 500 nM (or whatever makes sense given Kd and bead selection)
Peptides vs. proteins
A. Proteins
- Usually moreexpensive (if bought commercially, or in terms of time and resources required to clone, transfect, express, purify etc)
B. Peptides
- Generally less expensive (bought synthesized) – you can increase the concentration of the peptide to try to push equilibrium to AB complex
- Can control degree of labeling (i.e., can put one biotin on one end specifically during synthesis)
Determining Kd with an Alpha assay
Visit our page on using Alpha to determine binding affinity.
Cell-based protein-protein interaction assays
View our detailed information on performing cell-based interaction assays in Alpha. While performing biochemical assays is the most straightforward approach to study protein-protein interactions, oftentimes the cellular environment can be essential to generate the proper interaction.
Citations
View a brief listing of citations for Alpha biomolecular interaction assays.
Tips
- For biomolecular interaction assays, you might consider using weaker affinity beads (Ni-NTA and glutathione beads, rather than anti-GST and anti-His antibody beads) to bind your tagged biomolecules. There is less risk of hooking on the weaker affinity beads.
- You will need to run appropriate controls – this usually includes both beads and binding partner A (omitting partner B), then both beads and binding partner B (omitting partner A).
- We recommend you start with 20 µg/mL of each bead in your reaction. Bead concentration can be titrated later in your assay development (in a cross-titration matrix, titrating each bead from 10 µg/mL to 40 µg/mL).
- The theoretical maximum capacity of a streptavidin-coated bead at 20 µg/mL of bead is 30 nM. Please note that this does not take into consideration the size of the biotinylated molecule that will associate with the bead. For example, you may find that a biotinylated antibody will saturate the bead at 2-3 nM, rather than at 30 nM. If you have saturated the bead, you may see a hook effect.
- The theoretical maximum capacity of an antibody-coated bead is 3--10 nM. Please note this is a theoretical number — you may be able to add more or less than 3-10 nM "antigen" to your assay before hooking depending on how strong the antibody-antigen interaction is.
- The theoretical maximum capacity of a Ni-NTA or glutathione bead for a tagged protein is 3-30 nM, depending on the size of your protein. However, because the His-Ni-NTA interaction and the GST-glutathione interaction are weak (relative to biotin:streptavidin interaction, for example), you may be able to use more than 30 nM tagged protein (possibly up to 100 nM or more) before saturating the bead.
Troubleshooting
View our general Alpha troubleshooting tips.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























