Overview
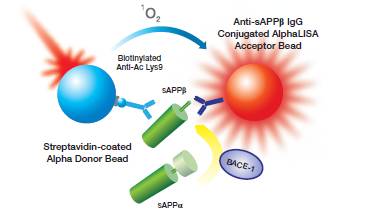
Our Alpha technology can be used to monitor protease cleavage of whole protein and peptide substrates. The traditional format uses two sandwiching antibodies, where one generic antibody binds the substrate and the other antibody specifically recognizes the neo-epitope created by the cleavage reaction. One of the antibodies associates with an Alpha Donor bead, while the other one associates with or is directly-conjugated to an AlphaLISA™ Acceptor bead. When the beads are brought together by the presence of cleaved product, laser excitation of the Donor bead will transfer energy to the Acceptor bead, resulting in the emission of light. This set up provides an increase in signal (with increasing protease activity) as depicted in Figure 1.

Figure 1. BACE protease assay in Alpha format using sandwiching antibodies, one of which recognizes the neo-epitope formed upon cleavage. Cleavage of the substrate results in an increase in signal.
If no antibody specific to the cleavage site is available, sandwiching antibodies can be used to monitor a splitting of the substrate, resulting in a decrease in signal with increasing protease activity. When studying peptide substrates, you can use an N-terminally biotinylated peptide with a streptavidin Donor bead, with an antibody that recognizes the C-terminal end associating with the AlphaLISA Acceptor bead. Alternatively, you can use different tags at the N- and C-termini with anti-tag antibodies on the Donor and Acceptor beads, etc. These assays will exhibit a decrease in signal assays with increasing protease activity (Figure 2).
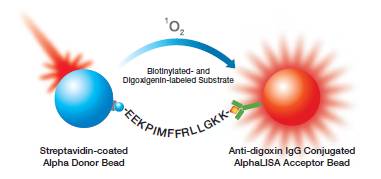
Figure 2. Cathepsin-D protease assay in Alpha format, using a peptide substrate that is biotinylated at one end and labeled with Dig at the other end, a streptavidin-coated Donor bead, and an anti-DIG-coated Acceptor bead. Cleavage of the substrate results in a decrease in signal.
What do I need to run this assay?
Required reagents available from Revvity:
- An appropriate Alpha Donor bead (see the list of available beads in Alpha reagents and catalog numbers below) .
- An appropriate AlphaScreen™ or AlphaLISA™ Acceptor bead (see the list of available beads in Alpha reagents and catalog numbers below).
- Microplates - We recommend our 96-well 1/2 AreaPlates or our 384-well white OptiPlates™. Also see Microplate selection.
- TopSeal™-A adhesive plate seal for incubations
Required reagents available from various suppliers:
- Protease
- Protease substrate
Instrumentation/equipment:
- A plate reader capable of reading Alpha assays (see Instrument options on the Alpha main page)
Assay development
For detailed information on assay design and development, view our Create your own Alpha assay page.
Citations
View a brief list of citations for Alpha protease assays.
Tips
- The neo-epitope assay format is usually the easiest to work with in terms of assay development. This format allows you to see an increase in signal with increasing protease activity. However, it requires an antibody that will recognize the neo-epitope formed when the substrate is cleaved.
- We recommend you start with 20 µg/mL Donor beads and 20 µg/mL Acceptor beads in your reaction. Bead concentration can be titrated later in your assay development (in a cross-titration matrix, titrating each bead from 10 µg/mL to 40 µg/mL).
- The theoretical maximum capacity of a streptavidin-coated bead at 20 µg/mL of bead is 30 nM. Please note that this does not take into consideration the size of the biotinylated molecule that will associate with the bead. For example, you may find that a biotinylated antibody will saturate the bead at 2-3 nM, rather than at 30 nM. If you have saturated the bead, you may see a hook effect.
- The theoretical maximum capacity of an antibody-coated bead is 3-10 nM. Please note this is a theoretical number — you may be able to add more or less than 10 nM "antigen" to your assay before hooking depending on how strong the antibody-antigen interaction is.
Troubleshooting
View our general Alpha troubleshooting tips.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























