
Assay principle

DELFIA™ (dissociation-enhanced lanthanide fluorescence immunoassay) is a time-resolved fluorescence (TRF) intensity technology. Assays are designed to detect the presence of a compound or biomolecule using lanthanide chelate labeled reagents, separating unbound reagent using wash steps. DELFIA assays are flexible, compatible with a variety of plate readers, and, as this is a wash-based technology, compatible with most sample types. The technology is based on fluorescence of lanthanide chelates (Europium, Samarium, and Terbium). The fluorescence decay time of these lanthanide chelate labels is much longer than traditional fluorophores, allowing efficient use of temporal resolution for reduction of autofluorescent background. The large Stokes’ shift (difference between excitation and emission wavelengths) and the narrow emission peaks contribute to increasing signal-to-noise ratio. Sensitivity is further increased because of the dissociation-enhancement principle: the lanthanide chelate is dissociated and a new highly fluorescent chelate is formed into a protective micellar solution. DELFIA lanthanide chelates require this dissociation/enhancement step for fluorescence (induced by addition of DELFIA Enhancement solution, DELFIA Inducer, and DELFIA Enhancer as appropriate to the particular lanthanide chelate).
Featured Products
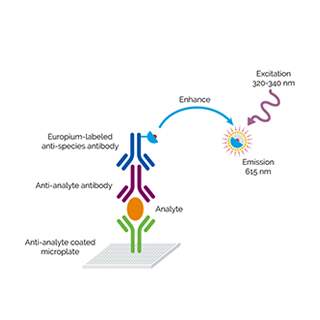
DELFIA TRF Assay
DELFIA™ TRF technology combines the benefits of Time Resolved Fluorescence (TRF) with the use of lanthanide chelates for a highly sensitive, robust and reproducible assay format.

Microplate Readers
As many of you know, microplate readers are instruments used to detect biological, chemical, biochemical or physical events from samples in a microplate.
Microplates for Research
We know you are working hard to produce the next big breakthrough and the last thing you need to worry about is sub-par quality in something so commonly used, yet often overlooked as a microplate.
Assay features
- Wash-based assay
- 96- and 384-well formats
- Filtration or solid-phase (microplate) assays
- Biochemical, cell membrane-based, and cell-based (live or fixed cell) assays
- Compatible with complex matrices, such as whole cells, cell membranes, cell lysates, tissue homogenates, serum, plasma, etc.
- Can be used for screening
- Multiplexing capability
Applications
- DELFIA immunoassays
- DELFIA cell cytotoxicity assays
- DELFIA cell proliferation assays
- Label your own DELFIA reagents
- Multiplexing DELFIA assays
- DELFIA receptor-ligand binding assays
- DELFIA kinase assays
- DELFIA biodistribution
DELFIA buffers
Read more about our various DELFIA buffer recommendations and compositions.
Products and catalog numbers
View a listing of relevant products and catalog numbers for DELFIA assays.
Custom Assay Development Services
Instrument options and settings
DELFIA assays require a plate reader that is capable of TRF (time-resolved fluorescence) detection. This is a time-resolved fluorescence intensity assay. We recommend using a Revvity VICTOR™ Nivo, VICTOR™ X, EnSight™, EnVision™, or EnSpire™ Multilabel Plate Reader (view our instrument product pages). If you are using a plate reader manufactured by another company, you can use our Europium standard solution (catalog number B119-100) and perform a serial dilution in DELFIA Enhancement solution to determine the sensitivity of your instrument. We also offer Samarium standard solution (catalog number B115-100) and Terbium standard solution (catalog number C558-100) if you are interested in multiplexing.
-
Fluorometers (filter fluorometer) vs. spectrofluorometers/monochromators
We recommend a time-resolved filter-based fluorometer for DELFIA Europium assays. Very sensitive time-resolved spectrofluorometers may be suitable for certain assays. Most plate readers are capable of TRF-type assays - a flash lamp is needed for TRF analysis, which is an option on most plate readers. You could also use laser excitation on an appropriate EnVision instrument.
- A TRF filter fluorometer plate reader utilizes bandwidth filters in front of the fiber optic for excitation and in front of the detector for emission wavelength choice. The grating-type spectrofluorometers are generally less sensitive than filter fluorometers, and loss in sensitivity can cause issues in highly sensitive assays such as TRF.
- A TRF-capable spectrofluorometer utilizes a monochromator (usually an excitation and emission grating) to discriminate wavelength positions of study for fluorescence excitation and emission. Spectrofluorometers can be up to 1000-fold less sensitive than filter fluorometers, though spectrofluorometers may work in some DELFIA applications.
- The new double-monochromator instruments claim to have almost similar sensitivity as the ‘original’ filter readers. These may be suitable for most DELFIA assays.
- For cell-based assays, illumination is recommended, as some clear-bottom plates may absorb excitation at 340 nm. Illumination is naturally required with white and black plates.
-
DELFIA Instrument Settings when using DELFIA Europium
We recommend generic default instrument settings for all DELFIA assays. We always recommend you check with the manufacturer of your particular instrument to see if they have additional recommendations for DELFIA assays.
Table: Settings for Revvity instruments
| General | VICTOR Nivo | EnSight | EnVision | EnSpire | VICTOR X | |
|---|---|---|---|---|---|---|
| Excitation | 320 or 340 wide-banded filter | 320/75nm (HH35000947) | UV (TRF340) dug11 + wg320 filter, barcode 101 (HH34005010) | UV (TRF340) dug11 + wg320 filter, barcode 101 (2100-5010) | UV (TRF340) dug11 + wg320 filter, (2300-5010) | 340/60 nm (built-in in VICTOR X4 and X5) |
| Emission | 615nm narrow filter bandwidth | 615/8nm (HH35000935) | 615nm (monochromator setting) | Europium 615/8.5 nm, barcode 203 (2100-5090) | 615nm (monochromator setting) | 616/8.5 nm (11440003) |
| Dichroic | D400 (HH35000971) | n/a | LANCE/DELFIA D400 single mirror, barcode 412 (2100-4170) | n/a | n/a | |
| Delay | 400µsec | 400µsec | 200µsec | 400µsec | 200µsec | 400µsec |
| Window | 400µsec | 400µsec | 400µsec | 400µsec | 400µsec | 400µsec |
| Other settings | Measurement time: 500ms | Number of Sequential Windows: 1 | Number of sequential windows: 1 | Number of Sequential Windows: 1 | Counting cycle: 1000µsec | |
| Flash energy: 100mJ | Number of Flashes: 100 | Number of flashes: 100 | Number of Flashes: 100 | Flash energy mode: High | ||
| Time between flashes: 2000µsec |
If you are working with Terbium and/or Samarium, please view the measurement information on our DELFIA multiplexing page.
Tips and Troubleshooting
You can find additional information under each specific Application (above).
- Allow DELFIA Enhancement solution warm to room temperature prior to use.
- If you are screening, we recommend that you shake your plates after adding Enhancement solution or Inducer to your wells. This will allow you to reach maximum, stable signal more-quickly. The recommended "slow" setting on a DELFIA plate shake is ~250 rpm with a shaking radius of approximately 3 mm. This is a fairly rigorous shake, though slow enough to avoid spillover.
- You must remove Seal™-A or any other plate seal prior to reading the assay.
- If you are adding BSA to your buffers for a DELFIA assay, we highly recommend you use our DTPA-purified BSA (catalog number CR84-100)
General FAQs
Q. Is the Europium radioactive?
A. No, this is not a radioactive isoe of Europium. DELFIA assays are non-radioactive fluorescence intensity assays.
Q. Can I use LANCE™ Europium reagents in a DELFIA assay?
A. You could. But you will still want to add Enhancement solution (or, ideally, Inducer) and dissociate the lanthanide to form a new chelate in solution. The reason for this is that though the LANCE Europium chelates are fluorescent without a dissociation step, they are not as bright as DELFIA Europium. In some heterogeneous assays, LANCE chelates can be used without dissociative enhancement. It is generally recommended, however, that an enhancement step be used in any DELFIA wash-based assay to increase the signal level and to get all chelates into solution to improve detection precision (even when you are using a LANCE Europium chelate in the DELFIA assay). The time for dissociation of LANCE Europium may depend greatly on the LANCE chelate used. Please consult with your drug discovery specialist or technical support if you plan to use a LANCE chelate in this manner. Note that some LANCE products have a lower Europium:biomolecule ratio (lower labeling ratio), which could affect sensitivity.
Q. Can I use DELFIA Europium reagents in a LANCE TR-FRET assay?
A. No, this is not possible. The DELFIA Europium chelate is not fluorescent until you add Enhancement solution. This dissociation step releases the lanthanide from the biomolecule it is attached to, and allows it to form a new chelate that is highly fluorescent, floating free in solution. In a TR-FRET assay, you cannot allow the lanthanide to dissociate from the biomolecule it is attached to - it would ruin your FRET assay. You must use a LANCE Europium chelate in LANCE assays.
Q: What are optimal DELFIA settings?
A: The standard DELFIA Europium protocol has the following settings: delay 400 µs and counting 400 µs for filter fluorometers. There is little optimization accomplished by using anything different. If you are using Terbium or Samarium, please refer to our DELFIA multiplexing page for instrument settings information.
Q: How can I validate my instrument for DELFIA Europium assays?
A: We have 1 nM Eu standard solution (catalog number B119-100). Expected counts are indicated on the technical data sheet. We also have standard solutions for DELFIA Terbium and Samarium.
Q: Can I get BSA-free or dextran-free DELFIA reagents?
A: We do get this question fairly frequently. Several of our DELFIA products and kits can be made available in special formulations. Please inquire with custom services.
Q: Why shouldn’t I use phosphate-based buffers when I add the Europium-labeled reagent?
A: Tris-HCl-based assay buffers work better in DELFIA assays than phosphate-based buffers, because maximum signal is lower with phosphate buffer. High concentrations of phosphate may potentially dissociate the Eu ion from the N1 chelate if a long incubation time is used. The DELFIA DTPA chelate is not affected by phosphate buffers.
Q: How long does it take for signal to develop?
A: When DELFIA Enhancement solution is added to the wells, the rate of signal enhancement depends on shaking. When DELFIA Eu-N1-labeled reagents are used, enhancement without shaking takes more than 1 hour. Shaking using DELFIA Plateshake (slow setting) gives maximum signal in 5 minutes. Most shakers are not as efficient as DELFIA Plateshake and give slower enhancement. This is easy to test: shake for 5 min, measure, shake for an additional 5 minutes, measure, etc. DELFIA Enhancement solution should reach room temperature before use because lower temperatures give higher counts, which could cause a temperature gradient in the plate. As a rule of thumb, a 1°C increase in temperature gives 1% lower counts.
Q: Can I stop at some point in my immunoassay?
A: We strongly recommend not incubating Enhancement solution overnight in the wells. DELFIA assays can be sped after final washing (before addition of Enhancement solution) and your plate can be stored for 1 day, 1 week, 1 year, 10 years, etc. when protected from dust by putting on a plate lid. If Enhancement solution has already been added and the plate can't be measured: 1) protect plate from dust (lid on BUT NO TAPE when Enhancement solution is in the wells); 2) Allow solution to completely evaporate; 3) Add Enhancement solution when ready, to regain signal.
Q: What sort of background counts should I expect in a cell-based assay?
A: Background using Revvity readers in DELFIA Eu measurement is about 100-200 counts assuming use of clean clear TC plates. When cells are cultured, fixed and incubated with Eu-labeled antibody then background is usually 1000-2000 counts. Normally, background in biochemical DELFIA assays (in vitro) is 300-1000 counts. One critical step when running DELFIA assays is washing after incubation with Eu-labeled reagent. Washing has to be efficient enough to remove unbound labeled reagent before the addition of DELFIA Enhancement solution. If cells are fixed, then a normal plate washer can be used. However, non-fixed cells require gentle washing.
Q: What is the difference between Enhancement solution, Inducer, and Enhancer?
A: DELFIA Enhancement solution is optimal for any N1 chelate (regardless of the lanthanide used). It dissociates the ions quickly and allows fluorescence development for Eu and Sm simultaneously. When a more-stable labeling chelate is needed, we recommend using DTPA chelates for labeling. Inducer provides a more rapid dissociation tool for DTPA labels. DTPA-labeled DELFIA reagents could also be enhanced with Enhancement solution, though this would require a longer dissociation time (30 minutes with shaking).
When either Eu or Sm is used as the label, the signal can be measured directly from Enhancement Solution, or Inducer. When Tb is used as an additional label, it is dissociated with Enhancement Solution or Inducer as described above, but also requires the addition of Enhancer to create a highly fluorescent Tb chelate.
DELFIA Immunoassay Tips
|
Assay Step |
Do |
Don't |
|---|---|---|
|
General |
Allow reagents to reach room temperature (20 to 25°C) before performing an assay. |
Don't use microplates with high fluorescent background. |
|
Avoid europium contamination and resulting high fluorescent background through careful pipetting and washing techniques. |
|
|
|
Use optimized DELFIA assay buffers to assure good results. |
|
|
|
Tracer |
Store labeled proteins and peptides at high concentration and in the absence of chelators or competing metals in the buffer. In most cases, 50 mM Tris-HCl buffer saline (pH 7.5-8.0) containing 0.1-0.5% purified BSA will ensure stability of the labeled compound during storage. |
Don't expose the tracer to chelating agents like EDTA. |
|
Avoid carry-over when pipetting a tracer solution by holding the pipet tip slightly above the of the well and avoiding touching the plastic strip or the surface of the liquid. |
Don't store labeled proteins or peptides in DELFIA assay buffer or phosphate buffers. |
|
|
|
Don't store diluted reagents. |
|
|
Enhancement |
Use 100-200 µL of Enhancement solution for 96-well and 50 µL for 384-well plates. |
Don't dispense Enhancement solution from labware which might have been contaminated with Europium. |
|
Pour the required amount of Enhancement solution (or Inducer) into a 15-mL or 50-mL conical tube. The conical tube and tip for dispensing should be dedicated for this purpose, and should be stored properly in a sealable plastic bag to prevent contamination. |
|
|
|
Use a dedicated Eppendorf Multipipette or another pipetting instrument for the Enhancement solution and discard the first aliquot. |
Don't seal the plate with tape after the addition of the Enhancement solution. The adhesive might quench the signal. |
|
|
Dispense the Enhancement solution slowly to avoid air bubbles. |
Don't use the same reservoir for Enhancement solution and tracer. |
|
|
Flush pipet or dispenser tips and tubing thoroughly with DELFIA Enhancement solution prior to use. |
|
|
|
Protect the plate from dust with a plate lid. |
|
|
|
Add the Enhancement solution just prior to shaking and measuring the plate |
|
|
|
Washing and shaking |
Optimize the number of wash cycles. |
Don't shake too vigorously, which may cause air bubbles that interfere in the fluorescence measurement. |
|
Use an automatic plate wash for optimal results. |
|
|
|
Ensure that each well is filled up completely to the edge. |
|
|
|
Check that the wells are dry after washing. If not, invert the plate and tap it firmly against absorbent paper. |
|
|
|
Check the time needed for efficient enhancement of lanthanide fluorescence for each shaker model. |
|
|
|
Measurement |
Measure with a sensitive time-resolved fluorometer like a VICTOR™ or EnVision™ |
Don't measure plates with their plate covers. |
DELFIA Citations
View a brief selection of DELFIA citations.
Custom labeling and assay development services
Revvity offers custom labeling services as well as custom assay development.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.





































