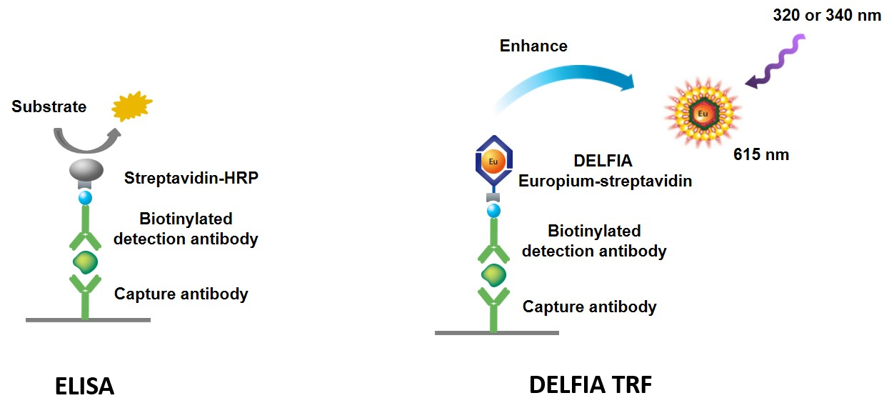
Overview
DELFIA™ immunoassays are a good alternative to traditional ELISAs (enzyme linked immunosorbent assays). The term ELISA originated during a time that this kind of assay setup needed an enzyme to generate the signal. In DELFIA ELISAs (DELFIA immunoassays), the use of an enzyme is not necessary. DELFIA assays can also be multiplexed.

Figure 1. Comparison of ELISA vs. DELFIA assay design.
Lanthanide-labeled immunoreagents can be applied in non-competitive (sandwich-type) or competitive time-resolved fluorescence assays. The design of each type of assay depends on the analyte and antibodies, and the required sensitivity and dynamic range. When the immunoreaction is complete, the Europium (Eu) ion is dissociated from the labeled immunocomponent (antibody, hapten, protein) bound to the solid phase by adding Enhancement solution. Then the Europium fluorescence is measured by time-resolved fluorometry (TRF).
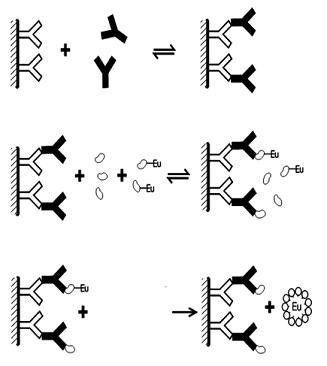
Figure 2. Example of a competitive cAMP assay in DELFIA format, using an anti-species antibody coated plate, an anti-cAMP antibody, and a Europium-labeled cAMP tracer.
DELFIA immunoassays can be performed in 96- and 384-well formats. You can coat antibodies or antigens directly or indirectly to DELFIA plates. Sandwich antibody assays, bridging/double-antigen assays, and fixed-cell assays have also been performed. You can use labeled antibodies, labeled antigens, or various labeled affinity reagents for the assay. DELFIA assays can also be used for immunogenicity studies.
- Competitive Assay:
- The Europium-labeled analyte (known) competes against possible analytes from your sample. The more (unknown) analyte present in your sample, the lower your signal will be. The less analyte in your sample, the higher your signal will be, as the Europium-labeled analyte will have no competition binding to your capturing site.
- After the Eu-analyte and sample analyte are left to compete against the capturing site (incubation), you wash away your unbound analyte. Only analyte bound to the capturing site remains in your well.
- Addition of Enhancement solution will then release Europium so that it can form a bright enhancing chelate, which you can then measure through TRF.
- Non-competitive Assay or Sandwich Assay:
- In a non-competitive assay, you will capture the analyte of interest between two capturing sites, usually antibodies. In this setup, one of the antibodies (instead of the analyte) will be labeled with the Europium. There will be no competition between known, labeled analyte and unlabeled sample analyte: if there’s no analyte in your sample, you will not have any signal, as the Europium-labeled antibody will not have anything to capture.
Animation of a DELFIA immunoassay. This immunoassay uses an antibody-coated plate to capture target analyte in the sample. The wells are then washed, and a DELFIA Europium-labeled antibody is added. After final washes, DELFIA Enhancement Solution is added to dissociate the Europium and allow it to form a new, highly fluorescent chelate in solution. The Europium chelate is excited at 320 or 340 nm, and fluorescence is measured at 615 nm.
The principle starts with a solid phase capturing site. When adding sample containing analyte, the analyte will be captured by the solid phase (i.e. well-bound) capturing site. Upon addition of Europium-labeled anti-analyte antibody, it will bind to the (now well-bound) analyte. Free Europium-labeled antibody is washed out of the well. Addition of Enhancement solution enables the TRF measurement of the Europium present. Other than with the competitive assay setup, your signal will behave in direct relation to the amount of analyte present: high analyte present results in high signal, whereas no analyte present results in no signal.
* Note: Instead of directly using a Europium-labeled anti-analyte antibody, you can also use a specific anti-analyte antibody, which then gets captured by a secondary Europium labeled antibody. Usually this is a more general, more common and less expensive antibody. This is an indirect setup: your labeled antibody labels your analyte indirect, i.e. by labeling the primary anti-analyte antibody.
What do I need to run this assay?
Required reagents available from Revvity:
- A Europium-labeled (or other DELFIA lanthanide-labeled) reagent for detection (view information on labeling your own reagent; we also offer standard Europium-labeled catalog products - see the list in the next section, under Products and Catalog numbers)
- A microplate indicated for fluorescence (96-well or 384-well - we recommend our DELFIA microplates)
- An appropriate wash buffer (we recommend DELFIA Wash buffers)
- An appropriate assay buffer for adding reagents (we recommend DELFIA Assay buffers)
- DELFIA Enhancement solution, DELFIA Inducer, or DELFIA Enhancer as appropriate (see FAQs below for more information)
- Seal™-A adhesive plate seal (do not use adhesive seals once Enhancement solution is in the wells)
Instrumentation/equipment:
- A TRF-capable plate reader
- Optional: Plate shaker (we recommend our DELFIA Plateshake)
- Optional: Platewasher (we recommend our DELFIA Platewash)
Our DELFIA Eu-labeling kit, catalog number 1244-302, was developed for DELFIA immunoassays. This kit contains a Europium labeling reagent so that you can label your own biomolecule. The kit also contains small sizes of DELFIA Enhancement solution, DELFIA Wash buffer, DELFIA Assay buffer, a DELFIA Europium standard, and 2 uncoated plates that can be used for direct coating.
Products and catalog numbers
View a listing of all relevant DELFIA products and catalog numbers.
Protocols
General Starting Protocol (96-well format)
For streptavidin coated plates:
- Add 50 µL of serum, plasma, cells or supernatant including your analyte
- Add 50 µL biotinylated anti-analyte antibody (200 ng/well) + Eu-labeled anti-analyte antibody (100 ng/well)
- Incubate 2 hours at room temperature (slow shaking)
- Wash 4-6x with DELFIA Wash solution, 300 μL per wash
- Add 200 µL DELFIA Enhancement solution
- Incubate 15 min at room temperature (with shaking)
- Measure TRF
For direct-coated plates:
- Add 50 µL of serum, plasma, cells or supernatant including your analyte
- Add 50 µL Eu-labeled anti-analyte antibody (100 ng/well)
- Incubate 2 hours at room temperature (with slow shaking)
- Wash 4-6x with DELFIA Wash solution, 300 μL per wash
- Add 200 µL DELFIA Enhancement solution
- Incubate 15 min at room temperature (with shaking)
- Measure TRF
Typically, the total signal and sensitivity is better using plates directly coated with antibody rather than with streptavidin. However, antibody-consumption is significantly higher in direct coating (e.g. 1 µg of antibody/well) than in indirect coating (e.g. 200 ng of biotinylated antibody/well), and coating plates with secondary antibody or streptavidin may often be the only viable option due to cost or limited availability of a specific antibody.
General Starting Protocol (384-well format)
For streptavidin coated plates:
- Add 25 µL of serum, plasma, cells or supernatant including your analyte
- Add 25 µL biotinylated anti-analyte antibody (50 ng/well) + Eu-labeled anti-analyte antibody (25 ng/well)
- Incubate 2 hours at room temperature (slow shaking)
- Wash 4-6x with DELFIA Wash solution, 100 μL per wash
- Add 50 µL DELFIA Enhancement solution
- Incubate 15 min at room temperature (with shaking)
- Measure TRF
Plate coating protocols
Direct Antibody Coating
This protocol is provided as an example protocol for plate coating. Other plate coating protocols could be used as well.
- Treat the wells with 100 µL of 10 µg/mL antibody prepared in 0.2 M sodium phosphate buffer at pH 6.8.
- Incubate 20 hours at room temperature.
- Wash wells 2-3 times with 1X PBS.
- Saturate wells with 200-250 µL saturation buffer [50 mM sodium phosphate pH 6.8, 6% trehalose (Sigma T9531), 0.1% BSA (Revvity, catalog number CR84-100), 0.1% Germal II (Vendico Chemical AB)] and incubate overnight at room temperature.
- Aspirate and store plate humid (aspirate and cover with Seal-A). Store at 4°C until use.
- If you would like to desiccate your plates, place in laminar flow hood 4-24 hours. Seal in foil and add desiccant bag. Store at 4°C.
- When plates are coated with 1 µg of antibody per well, then 150-250 ng of biologically active antibody is normally coated on the well. Some of the antibody is coated in a way so that it’s not capable of binding.
- Note: When antibodies are coated, it is good to remember that some antibodies are difficult to coat. Some can lose most of their biological activity during the coating process. In these cases, anti-species coated plates or streptavidin coated microplates have to be used.
- There are many different grades of BSA, and some of these may contain a considerable amount of heavy metals that will eventually show as high levels of background in the assay. Using purified BSA is highly recommended; alternatively, a high grade of casein or gelatine can be used for the saturation of the plates. Stabilizer, 7.5% purified BSA (prod.no. CR84-100) is available from Revvity.
Direct Antigen Coating
The important thing with antigen coating is the MW of the antigen. Many antigens with MW less than 50 kDa are very difficult to coat and should be immobilized by biotinylating them and using streptavidin coated microplates. However, sometimes larger antigens may also require the use of streptavidin coated plates. In general, the coating should be performed at room temperature or 4°C overnight. Testing of different pH levels (pH 4, pH 7 or pH 9) can be very useful. A 50 mM phosphate buffer containing 0.9% NaCl would be a good antigen coating buffer.
Indirect Antibody Coating
As stated before, some antibodies are very hard to coat to plates or even lose their biological activity during the coating process. The workaround can be the use of our pre-coated plates. We have DELFIA anti-species plates, as well as DELFIA streptavidin coated plates for use with a biotinylated antibody. There are commercially available biotinylation kits (e.g. from Pierce or Solulink).
Protocol for fixing cells to a plate
Suggested cell fixing protocol
Assay Optimizations
1. Microplate selection
- The plate coating (pre-coated indirect capture plates, or direct coating of high binding plates)
- The fluorescence compatibility (our yellow DELFIA plates are optimized for DELFIA assays, particularly for multiplexing assays)
2. Capture antibody
- Selection
- Titration
3. Tracer
- Selection (directly-labeled reagents or indirect detection)
- Tracer titration
4. Assay conditions
- Assay buffer (we recommend our DELFIA Assay buffer)
- Incubation buffers for Europium-labeled ligands or other reagents should be Tris-based (50 mM, pH 7.5-8) containing 0.9% NaCl, 0.01-0.5% BSA, 0.001-0.05% Tween 20 or 40, and 20 µM EDTA. DELFIA Assay buffer would be ideal, since phosphate buffers tend to lower the DELFIA signal.
- Washing (we recommend thorough washing, 4-6 times at each step)
- Washing steps: thorough washing is crucial in the DELFIA ELISA assays. A majority of cases where high background is observed can be solved by increasing the number of washing steps. The general recommendation would be 4x washing, per washing step in the protocol. But for some assays and setups, this can be as high as 6 to 8 times in order to get acceptable signal windows.
Application notes
- The Guide to converting ELISA Assays to DELFIA - contains detailed information on how to convert from ELISA to DELFIA, DELFIA assay optimization steps, recommended concentrations of DELFIA reagents to use in the assay, general DELFIA tips and troubleshooting information
- How to optimize rapid and simple immunoassays
- DELFIA immunogenicity testing
- Poster for detecting phospho-p44/42 MAPK in fixed cells
Tips and FAQs
- Allow DELFIA Enhancement solution warm to room temperature prior to use.
- If you are screening, we recommend that you shake your plates after adding Enhancement solution or Inducer to your wells. This will allow you to reach maximum, stable signal more quickly. The recommended "slow" setting on a DELFIA plate shake is ~250 rpm with a shaking radius of approximately 3 mm. This is a fairly rigorous shake, though slow enough to avoid spillover.
- You must remove Seal™-A or any other plate seal prior to reading the assay.
- If you are adding BSA to your buffers for a DELFIA assay, we highly recommend you use our DTPA-purified BSA (catalog number CR84-100)
DELFIA Immunoassay Tips
| Assay Step | Do | Don't |
|---|---|---|
| General | Allow reagents to reach room temperature (20 to 25°C) before performing an assay. | Don't use microplates with high fluorescent background. |
| Avoid europium contamination and resulting high fluorescent background through careful pipetting and washing techniques. | ||
| Use optimized DELFIA assay buffers to assure good results. | ||
| Tracer | Store-labeled proteins and peptides at high concentration and in the absence of chelators or competing metals in the buffer. In most cases, 50 mM Tris-HCl buffer saline (pH 7.5-8.0) containing 0.1-0.5% purified BSA will ensure stability of the labeled compound during storage. | Don't expose the tracer to chelating agents like EDTA. |
| Avoid carry-over when pipetting a tracer solution by holding the pipet tip slightly above the well and avoiding touching the plastic strip or the surface of the liquid. | Don't store labeled proteins or peptides in DELFIA assay buffer or phosphate buffers. | |
| Don't store diluted reagents. | ||
| Enhancement | Use 100-200 µL of Enhancement solution for 96-well and 50 µL for 384-well plates. | Don't dispense Enhancement solution from labware which might have been contaminated with Europium. |
| Pour the required amount of Enhancement solution (or Inducer) into a 15-mL or 50-mL conical tube. The conical tube and tip for dispensing should be dedicated for this purpose and should be stored properly in a sealable plastic bag to prevent contamination. | ||
| Use a dedicated Eppendorf Multipipette or another pipetting instrument for the Enhancement solution and discard the first aliquot. | Don't seal the plate with tape after the addition of the Enhancement solution. The adhesive might quench the signal. | |
| Dispense the Enhancement solution slowly to avoid air bubbles. | Don't use the same reservoir for Enhancement solution and tracer. | |
| Flush pipet or dispenser tips and tubing thoroughly with DELFIA Enhancement solution prior to use. | ||
| Protect the plate from dust with a plate lid. | ||
| Add the Enhancement solution just prior to shaking and measuring the plate | ||
| Washing and shaking | Optimize the number of wash cycles. | Don't shake too vigorously, which may cause air bubbles that interfere in the fluorescence measurement. |
| Use an automatic plate wash for optimal results. | ||
| Ensure that each well is filled up completely to the edge. | ||
| Check that the wells are dry after washing. If not, invert the plate and tap it firmly against absorbent paper. | ||
| Check the time needed for efficient enhancement of lanthanide fluorescence for each shaker model. | ||
| Measurement | Measure with a sensitive time-resolved fluorometer like a VICTOR™ or EnVision™ | Don't measure plates with their plate covers. |
Q. How much labeled Europium antibody should I add?
A. Usually, the amount of labeled antibody needs to be optimized when developing a DELFIA assay. As a general rule, 25-100 ng/well of labeled antibody is enough for noncompetitive sandwich-type of assay, but the actual optimal level depends on the purity and affinity of the antibody and the signal level desired.
Q. Can I use whole cells?
A. Yes. You can use normal tissue culture-treated plates indicated for fluorescence. Basically, any color is okay, though common colors are white and clear. We usually recommend opaque white CulturPlates™. You can use fixed cells (protocol for fixing cells). The critical things in a DELFIA assay with adherent live cells include incubation buffer with Eu-labeled reagent and washing after incubation with Eu-labeled reagent. Assay buffer for Eu-labeled reagent should be Tris-HCl (50 mM) or HEPES buffered solution containing 0.9% NaCl (and possibly some other salts), 0.1-0.5% BSA, and 20 µM EDTA or DTPA. Suitable concentration for Eu-labeled antibody is 100-300 ng/mL during incubation. Suitable wash solution is 10-20 mM Tris-HCl pH 7.5 containing 0.9% NaCl. Washing must be performed gently and should include 4-8 wash cycles. If washing is performed manually, then each wash should be 300-350 µL/well (in 96-well format).
-
For fixed cells, a good reference:
Hardcastle, A. et al. A Duplexed Phenotypic Screen for the Simultaneous Detection of Inhibitors of the Molecular Chaperone Heat Shock Protein 90 and Modulators of Cellular Acetylation." Mol Cancer Ther 6, 1112-1122 (2007).
Q: Can I stop at some point in the assay?
A: We strongly recommend not incubating Enhancement solution overnight in the wells. DELFIA assays can be sped after final washing (before addition of Enhancement solution) and your plate can be stored for 1 day, 1 week, 1 year, 10 years, etc. when protected from dust by putting on a plate lid. If Enhancement solution has already been added and the plate can't be measured: 1) protect plate from dust (lid on BUT NO TAPE when Enhancement solution is in the wells); 2) Allow solution to completely evaporate; 3) Add Enhancement solution when you are ready, to regain signal.
Q: Why can’t I use phosphate-based buffers when I add the Europium-labeled reagent?
A: Tris-HCl-based assay buffers work better in DELFIA assays than phosphate-based buffers, because maximum signal is lower with phosphate buffer. High concentrations of phosphate may potentially dissociate the Eu ion from the N1 chelate if a long incubation time is used. The DELFIA DTPA chelate can stand phosphate buffers.
Q: What is the difference between Enhancement solution, Inducer, and Enhancer?
A: DELFIA Enhancement solution is optimal for any N1 chelate (regardless of the lanthanide). It dissociates the ions quickly and allows fluorescence development for Eu and Sm simultaneously. When a more-stable labeling chelate is needed, we recommend using DTPA chelates for labeling. Inducer provides a more rapid dissociation tool for DTPA labels. DTPA-labeled DELFIA reagents could also be enhanced with Enhancement solution, though this would require a longer dissociation time (30 minutes with shaking).
When either Eu or Sm is used as the label, the signal can be measured directly from Enhancement Solution, or Inducer. When Tb is used as an additional label, it is dissociated with Enhancement Solution or Inducer as described above, but also requires the addition of Enhancer to create a highly fluorescent Tb chelate.
Q: Will I have to add extra components to my assay buffer/wash solutions?
A: Certain types of assays will benefit from added detergents or the addition of extra immunological blockers. Casein and gelatins are commonly used immunological blockers.
Citations
View a brief list of DELFIA immunoassay citations.
Troubleshooting
|
Problem |
Cause |
Solution |
|---|---|---|
|
High background signal |
Background of the plain plate is >500 (no coating, no buffer, no Enhancement solution) |
Change the plates. |
|
Inadequate washing prior to measurement |
Use 4-6 washing cycles in a DELFIA Platewash after incubation with Eu-labeled compound. |
|
|
Inadequate blocking of the plates |
Saturate overnight at room temperature, or >2 hours at 37°C. |
|
|
Use a saturation volume greater than that of the coating solution. |
||
|
Binding of the Eu-labeled polyclonal antibody to the plate |
Add 0.01%-0.1% Tween 20 or Tween 40 to the assay buffer. |
|
|
Contamination of pipettes and tables with label |
Clean pipettes and tables carefully. |
|
|
Inadequate sensitivity |
High background |
See above. |
|
Low maximal signal |
Increase the sample volume. |
|
|
Use two different tracer antibodies. |
||
|
Increase the antibody concentration in a sandwich assay. |
||
|
Change the antibody used. |
||
|
Poor reproducibility |
Inadequate incubation with Enhancement solution |
Incubate for at least 5 minutes on a shaker before measurement. Check the time needed for maximum signal. |
|
Antibody aggregation |
Filter the antibody or the antibody diluted in assay buffer through 0.22 µm filter. |
|
|
Low affinity of antibody |
For immunometric assays, increase the amount of tracer per well or the incubation time, check the coating procedure, etc. |
|
|
Uneven coating |
Test with Revvity microplates. |
|
|
Plate sealed with tape during the enhancement and measurement steps |
Do not cover the plate containing Enhancement solution with tape. |
|
|
Trace amounts of Eu in sample |
Add 20 µM DTPA or EDTA to the assay buffer. |
|
|
Labeled antibody stored at wrong temperature |
Optimize the storage conditions. |
|
|
Decrease in assay counts after storage of labeled compound |
Heavy metal contamination of BSA |
Add purified stabilizing agents like glycerol, glucose, or BSA (catalog number CR84-100). |
|
Unsuitable storage conditions |
Do not expose the Eu-labeled antibody to chelating agents like EDTA or phosphate-based buffers during storage. Store the Eu-antibody undiluted in Tris-HCl buffer (50 mM Tris-HCl, 0.9% NaCl, 0.05% NaN3, pH 7.8). |
|
|
Instability of antibody or other reagents |
Optimize the reagents. |
Custom labeling and assay development services
Revvity offers custom labeling services as well as custom assay development.
DELFIA diagnostic kits
If you are using one of our DELFIA diagnostic kits, please contact the genetic screening technical support teams for assistance
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























