Overview
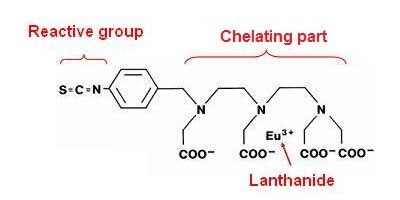
Chelate structure
DELFIA™ chelates can be broken down into three parts:
1. The reactive group. The reactive group dictates what chemical group on your sample will be conjugated/labeled.
- ITC (isothiocyanate) reacts with free amines, including the N-terminus of a protein or peptide, and lysine residues.
- Iodoacetamide reacts with free sulfhydryl groups (cysteines).
- Amino reacts with free carboxyl groups.
- DTA (dichlorotriazine) reacts with free amino groups, sulfhydryl groups, and hydroxyl groups. Because of its high reactivity, we usually do not recommend DTA as it may “inactivate” the reagent you are labeling.
2. The chelating part. The chelating part coordinates to the lanthanide ion, and dictates properties such as chelate stability.
- N1: easy labeling, short dissociation time, best suited for assay conditions where concentration of EDTA is less than 0.05 mM, pH is greater than 7, and assay temperature does not exceed 38°C.
- DTPA: more stable, harder to dissociate (recommend using DELFIA Inducer instead of Enhancement solution), can be used in assays with concentration of EDTA up to 10 mM, pH greater than 5.0, and temperatures up to 100°C.
3. The lanthanide. There are three lanthanides for DELFIA.
- Europium
- Samarium
- Terbium

Chemical structure of the Eu-labeling reagent, N1-(p-isothiocyanatobenzyl)-diethylene-triamine-N 1,N 2,N 3,N 3-tetracetic acid chelated with Eu 3+.
DELFIA chelates, dissociation, and enhancing the signal
All DELFIA labeling chelates require dissociation from their N1 or DTPA chelate and enhancement for fluorescence. The intrinsic fluorescence of the N1 and DTPA chelates is very low. Dissociation is accomplished using DELFIA Enhancement Solution or DELFIA Inducer. Additionally, DELFIA Enhancer is required to enhance the signal for Terbium chelates. Because DELFIA chelates require dissociation, these chelates cannot be used in FRET or time-resolved microscopy applications (LANCE Europium would be used instead).
- DELFIA Enhancement solution is for Europium and Samarium N1 chelate dissociation and enhancement, as well as for Terbium N1 chelate dissociation
- DELFIA Inducer is a more-strongly dissociating solution recommended for Europium and Samarium DTPA chelates, as well as for Terbium DTPA chelate dissociation
- DELFIA Enhancer is for Terbium enhancement, after dissociation has been first accomplished with Enhancement solution or Inducer
Shaking Times for the DELFIA Plateshake to Reach 98% of Maximum Signal
|
Chelate |
Shaking time with Enhancement solution |
No-shaking time with Enhancement solution |
Shaking time with Inducer |
No-shaking time with Inducer |
|---|---|---|---|---|
|
N1 |
5 min |
45 min |
5 min |
30 min |
|
DTPA |
30 min |
180 min |
5 min |
30 min |
* If you are screening, we recommend shaking your plates after adding Enhancement solution or Inducer. This will allow you to reach maximum, stable signal more- quickly.
DELFIA Europium chelate table
N1 chelates: Easy labeling and purification, as well as short dissociation time. Best suited for DELFIA where concentration of chelating agents (such as EDTA) is less than 0.05 mM, where pH > 7 and assay temperature does not exceed 38°C.
DTPA chelates: More stable chelate, but requires longer dissociation time. Can be used in DELFIA assays where concentration of chelating agents (such as EDTA) is as high as 10 mM. Supports pH down to 5.0 and assay temperatures as high as 100ºC.
DELFIA Europium Chelate Table
|
Catalog number |
Product name |
Quantity |
Common sample type |
Reactive with |
MW of Europium chelate |
Residual MW to labeled molecule |
|---|---|---|---|---|---|---|
|
1244-302 |
DELFIA Europium labeling kit (Eu-N1-ITC chelate) |
0.2 mg |
Proteins/antibodies/peptides |
Free amino groups |
654 Da |
654 Da |
|
1244-301 |
Eu-N1 ITC chelate |
1 mg |
Proteins/antibodies/peptides |
Free amino groups |
654 Da |
654 Da |
|
AD0001 |
Eu-N1 ITC chelate |
20 mg |
Proteins/antibodies/peptides |
Free amino groups |
654 Da |
654 Da |
|
AD0002 |
Eu-N1-Iodoacetamido chelate |
1 mg |
Proteins/peptides |
Free sulfhydryl groups |
780 Da |
652 Da |
|
AD0003 |
Eu-N1-amino chelate |
1 mg |
Small molecules |
Carboxyl groups |
611 Da |
595 Da |
|
AD0004 |
Eu-N1-DTA chelate |
1 mg |
Peptides/small molecules |
Free amino groups, sulfhydryl groups, and hydroxyl groups |
760 Da |
724 Da |
|
AD0021 |
Eu-DTPA-ITC chelate |
1 mg |
Proteins/antibodies/peptides |
Free amino groups |
749 Da |
749 Da |
|
AD0023 |
Eu-DTPA-amino chelate |
1 mg |
Small molecules |
Carboxyl groups |
706 Da |
690 Da |
|
AD0024 |
Eu-DTPA-DTA chelate |
1 mg |
Peptides/small molecules |
Free amino groups, sulfhydryl groups, and hydroxyl groups |
855 Da |
819 Da |
DELFIA Samarium and Terbium chelate table
N1 chelates: Easy labeling and purification, as well as short dissociation time. Best suited for DELFIA where concentration of chelating agents (such as EDTA) is less than 0.05 mM, where pH > 7, and assay temperature does not exceed 38°C.
DTPA chelates: More stable chelate, but requires longer dissociation time. Can be used in DELFIA assays where concentration of chelating agents (such as EDTA) is as high as 10 mM. Supports pH down to 5.0 and assay temperatures as high as 100ºC.
DELFIA Samarium and Terbium Chelate Table
|
Catalog number |
Product name |
Quantity |
Common sample type |
Reactive with |
MW of Samarium or Terbium chelate |
Residual MW to labeled molecule |
|---|---|---|---|---|---|---|
|
1244-303 |
DELFIA Samarium labeling kit (Sm-N1-ITC chelate) |
0.2 mg |
Proteins/antibodies/peptides |
Free amino groups |
652 Da |
652 Da |
|
AD0009 |
Tb-N1-ITC chelate |
1 mg |
Proteins/antibodies/peptides |
Free amino groups |
661 Da |
661 Da |
|
AD0029 |
Tb-DTPA-ITC chelate |
1 mg |
Proteins/antibodies/peptides |
Free amino groups |
756 Da |
756 Da |
Guides
DELFIA Labeling guide
Labeling calculations
Math
Example A: A scientist has an monoclonal antibody, MW 160,000 Da. She wants to label 1 mg of antibody with the Eu-N1-ITC chelate (catalog number 1244-302). How much chelate would she need to add to her 1 mg of protein, to get a labeling yield of 8 Eu per antibody? The concentration of antibody in the labeling reaction will be 5 mg/mL.
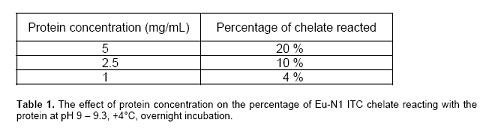
- You will need to use the reactivity chart (Table 1) for this chelate, as well as its molecular weight. Each chelate will have a different reactivity and molecular weight. Refer to the tables above for molecular weight information.
DELFIA chelate reactivity
- If 20% of the chelate is expected to react, and you would like to have an 8:1 labeling yield, you will need a 40X molar excess chelate over antibody.

DELFIA labeling math
Polyclonal antibodies tend to have a lower labeling efficiency so you would most often end up with 3-6 Eu/polyclonal Ab in this reaction - this is actually an ideal labeling ratio for polyclonal antibodies in DELFIA.
Example B: A scientist has 261 nmol of peptide, and wants to know how much chelate to add. Assume one lysine residue in the peptide. We recommend adding a 3-6 fold excess chelate over peptide if your peptide concentration is 5-20 mg/mL, a 5-10 fold excess chelate over peptide if your peptide concentration is 2.5--5 mg/mL, and an 8-30 fold excess chelate over peptide if your peptide concentration is 1-2.5 mg/mL.
- At a peptide concentration of 1.5 mg/mL, you can expect 20% reaction. So, if you want to aim for one label per molecule, and you have 261 nmol of peptide, you should add 1305 nmol of Europium chelate (to make a 5X excess; Eu chelate MW 654 comes out to 0.85 mg of Europium chelate).
- Most often, synthetic peptides are labeled at room temperature (only peptides should be labeled at room temperature). Proteins or antibodies should not be labeled at room temperature as they tend to lose their biological activity at high pH at room temperature.
Example C: R&D labeling calculation. How would Revvity R&D label RANKL peptide (MW 20,000)?
- RANKL (MW 20,000) has 19 acidic amino acid residues (Glu and Asp) and 17 basic residues (Lys and Arg) and this protein is slightly acidic (pI slightly below 7). There are 12 Lys residues in RANKL, meaning that labeling efficiency at normal labeling conditions (pH 9.3, 4°C, overnight) at RANKL concentration of 5 mg/mL is as follows:
- 5 mg/mL RANKL = 250 nmol/mL = 250 µM, consequently Lys residue concentration is 12 x 250 µM = 3 mM
- It is known that the reaction efficiency of Eu-N1-ITC chelate at pH 9.3, 4°C, overnight is 20% at Lys residue concentration of 1.5 mM.
- So the reaction efficiency of Eu-N1-ITC chelate at 5 mg/mL RANKL (3 mM Lys residue) is 40%, meaning that 40% of added chelate reacts with RANKL.
- Suitable target with a protein having MW of 20,000 is 1.5-2 Eu chelates per protein. If we take a target of 1.5 Eu chelates, then a suitable molar excess of Eu-N1-ITC over RANKL at 5 mg/mL is calculated in the following way:
- Molar excess = target/reaction efficiency = 1.5/0.40 = 3.8
-
So the above is valid when labeling is performed at 5 mg/mL RANKL and the molar excess for other RANKL concentrations can be calculated easily
(5 mg/mL)/RANKL concentration (mg/mL)) x 3.8
So at 1 mg/mL molar excess should be (5/1) x 3.8 = 19
Effect of isoelectric point, pI, on the labeling of proteins
Our chelates, N1 and DTPA, have a net negative charge. The majority of proteins will have a pI below the labeling pH (9.3). Under these conditions, both the chelate and the protein to be labeled have a net negative charge, resulting in an electrostatic repulsion. Consequently, when labeling is performed at pH 9.3, 4°C, overnight, given 1.5 mM concentration of Lys residues, then the labeling efficiency is 20%. However, labeling of proteins having a net positive charge at labeling pH is far more efficient.
FAQs
Q. Can I use dialysis to purify away free Europium?
A. Dialysis is not sufficient for removing free Europium. This is not recommended.
Q: Can I use spin columns to purify my labeled reagent?
A: We have tried to use spin columns in the past; however, the spin columns were not efficient enough to remove the unreacted. Desalting columns (PD-10 and NAP-25 from GE) work with N1 chelates to some extent. These columns contain Sephadex G-25. When working with these columns, keep the following restrictions in mind: First, no more than 120 nmoles of Eu-N1 should be applied to the column. Secondly, the volume of the labeling reaction should be fairly low, preferably 50-200 µL, but no more than 400 µL. After the reaction mixture has entered the column, fractions of 0.5 mL (TSA buffer for equilibration and elution, explained in the DELFIA Labeling guide) are collected, totally 15 fractions. Eu-labeled protein or peptide (MW at least 3 kDa) elutes roughly in the fractions 6-9. These ready-made desalting columns don't work with DELFIA DTPA or LANCE chelates. Please note that PD-10 and NAP-25 are not spin columns.
Q: Which lanthanide should I use?
A: We recommend lanthanides in this order: Europium, then Terbium, then Samarium. View more information on multiplexing.
Q: What is the difference between ITC- and DTA-activated chelates? They both react with primary amino groups…
A: ITC-activated chelates react with amino and sulfhydryl groups, but the reaction product with sulfhydryl groups decomposes immediately. DTA-activated chelates react with amino groups, sulfhydryl groups (forming a stable conjugate), tyrosine residues, and possibly with some other amino acid residues. DTA-activated chelates are 1.5-2 times more reactive than ITC-activated chelates when proteins are labeled. However, due to the fact that DTA-activated chelates react with several amino acid residues (Lys, Cys, Tyr, amino terminus) on the proteins, there is a danger of proteins becoming inactive upon labeling. ITC-labeling is safer.
Q. What labeling ratio should I aim for?
A. When labeling antibodies, generally about 6-12 Eu/IgG with monoclonal antibody is an optimal yield that gives high sensitivity with low background. For many assays, even a lower labeling yield gives acceptable results. For polyclonal antibodies, the suitable number of Eu chelates coupled is 3-5. Labeling of antibodies with over 20 Eu/IgG may occasionally cause aggregation and an elevated background, especially after storage. Proteins with a lower molecular weight should be labeled with fewer chelates than monoclonal antibodies. Proteins with molecular weight 30-70 kDa are preferably labeled with 2-6 chelates, and proteins with molecular weight less than 30 kDa with 1-3 chelates. The labeling yield needs to be optimized separately for each particular protein and the assay requirements. Monoclonal antibodies may especially behave individually.
Q: What is the difference between Enhancement solution, Inducer, and Enhancer?
A: DELFIA Enhancement solution is optimal for any N1 chelate (regardless of the lanthanide used). It dissociates the ions quickly and allows fluorescence development for Eu and Sm simultaneously. When a more-stable labeling chelate is needed, we recommend using DTPA chelates for labeling. Inducer provides a more rapid dissociation tool for DTPA labels. DTPA labeled DELFIA reagents could also be enhanced with Enhancement solution, though this would require a longer dissociation time (30 minutes with shaking).
When either Eu or Sm is used as the label, the signal can be measured directly from Enhancement Solution, or Inducer. When Tb is used as an additional label, it is dissociated with Enhancement Solution or Inducer as described above, but also requires the addition of Enhancer, to create a highly fluorescent Tb chelate.
Q: Instead of reconstituting the chelate first, can I directly add my protein-to-be-labeled into the vial containing lyophilized chelate?
A: Yes. Reconstituting the chelate in water or sodium acetate allows you to save a portion of unreacted chelate for future use, but you can do your labeling reaction directly in the vial of chelate if your protein is in the correct buffer for labeling (and molar excess chelate over protein/peptide is correct).
Q: Can I just use a portion of the Eu-labeling reagent for labeling and store the rest of the reagent?
A:
- N1-ITC chelates (Eu-N1-ITC, catalog numbers 1244-301 and 1244-302; Sm-N1-ITC, catalog number 1244-303, Tb-N1-ITC, catalog number AD0009) are dissolved just prior to use. If some of the dissolved chelate is to be used later, then the dissolved chelate has to be stored at -20°C or -70°C. To dissolve the lyophilized chelates, follow these instructions:
- Lyophilized chelates (catalog numbers 1244-301, 1244-302, and 1244-303) are dissolved in water. When stored in water, these chelates are stable for at least 4 weeks at -20°C.
- ITC-activated chelates in powder form (catalog number AD000X) are dissolved in water if used immediately. If some of the chelate is going to be stored for future use, then these chelates in powder form should be dissolved in 50 mM sodium acetate, pH 4.5-4.7. When stored in acetate buffer, these chelates are stable for 3-4 weeks at -20°C.
Q: How should I store my Eu-labeled material?
A: DELFIA-labeled reagents must be stored in Tris-HCl buffered solution containing sodium chloride, sodium azide (not necessary when stored at -20°C or -70°C) and metal-free BSA for stabilization (we recommend catalog number CR84-100, for BSA). When stored at a reasonable concentration (e.g. no less than 10 µg/mL for antibodies) in this buffer, DELFIA-labeled reagents are stable for up to 1-2 years. We do not recommend storing labeled material in phosphate-based buffers. Tris-HCl based assay buffers work better in DELFIA than phosphate- based buffers, because maximum signal is lower with phosphate buffer. Additionally, the higher the phosphate concentration in the assay buffer, the lower the signal. You should not store in DELFIA assay buffer, which contains 20 µM DTPA, a strong chelating agent. Labeled proteins and peptides should be stored at a high concentration and in the absence of chelators.
Q: Can I end-label my peptide? I think it will improve my assay.
A: If there is either a Ser or Thr at the N-terminus, then you can first oxidize, followed by a reaction with an aminooxy-activated (amino) chelate.
Reference
Peuralahti, J. et al. Synthesis of Nonluminescent Lanthanide(III) Chelates Tethered to an Aminooxy Group and Their Applicability to Biomolecule Derivatization. Bioconjug Chem 13, 876-880 (2002). Link
Q: Do I have to label at 4°C?
A: Only peptides (no more than 50 amino acids) can be labeled at room temperature. Temperature is very important when labeling antibodies, which consist of several polypeptide chains. Conditions for antibody labeling should be 4°C, pH 9.3, overnight. If an antibody doesn't stand overnight labeling, then a 4-hour protocol should be followed (using 3 times more chelate than in overnight labeling). Of course, reaction efficiency is higher at room temperature than at 4°C. However, if a protein loses its binding properties because it has been incubated in the labeling conditions (RT) overnight, then higher labeling efficiency doesn't bring any advantages.
Q: Do I have to label at pH 9.3? My antibody isn't very stable.
A. One possibility to avoid the problem of an overnight reaction at 4°C, pH 9.3, is to perform a 4-hour reaction (at 4°C, pH 9.3) using 3 times more chelate than in the overnight reaction. The other possibility is to label at pH 8.5-8.6, 4°C, overnight using 4 times more chelate than in the reaction at pH 9.3, 4°C.
Citations
View a brief listing of selected DELFIA labeling citations.
Custom labeling and assay development services
Revvity offers custom labeling services as well as custom assay development. If you are interested in having your biomolecule custom-labeled, or in custom assay development, please contact our custom teams:
- Custom Labeling and Conjugation Services
- Custom Assay Development Services
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























