Order-of-preference for labels
We recommend the lanthanides in this order: Europium (Eu), then Terbium (Tb), then Samarium (Sm). When choosing the labels, keep in mind that Samarium gives less signal (and may not give the same sensitivity, depending on background) than Terbium. For those who cannot compromise sensitivity, we recommend using Europium and Terbium – which, however, require the addition of an additional enhancer (Enhancement solution to dissociate the Europium and Terbium and to enhance the Europium for the Europium measurement, followed by addition of Enhancer to enhance the Terbium for the Terbium measurement). The advantage of choosing Europium and Samarium is that you only need Enhancement solution to both dissociate and enhance the signal for both labels.

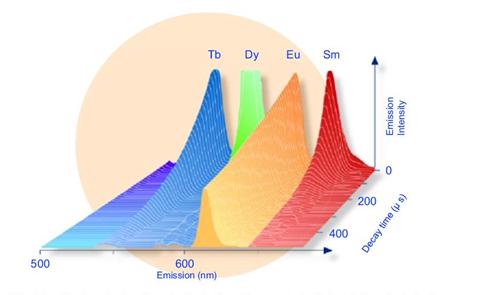
The chelates have been developed from three lanthanides with spectra clearly distinguishable on the basis of decay time and wavelength. The narrow emission peaks in different wavelengths are 613 nm for Europium (Eu), 643 nm for Samarium (Sm), 545 nm for Terbium (Tb).
General tips
The various lanthanides, Eu, Sm, and Tb, won't have the same sensitivity in DELFIA™ assays. Eu and Tb give roughly the same sensitivity, whereas Sm is measured with 100 times lower sensitivity. However, that difference can be narrowed down to 10-20 times lower sensitivity by using a higher concentration of Sm-labeled reagent. The background in Sm measurement at 643 nm is still lower than in the Eu measurement at 615 nm (200-250 counts versus 300-1000 counts). For example, typically one can use 200-500 ng/mL Eu-labeled antibody and 500-1500 ng/mL Sm-labeled antibody in sandwich immunoassays. Tb is measured at 545 nm after a long delay time, and the background tends to be fairly high (typically 1500-3000 counts depending on the plate).
DELFIA measurement is based on the use of DELFIA Enhancement solution (alternatively DELFIA Inducer can be used) and DELFIA Enhancer. DELFIA Enhancement solution and DELFIA Inducer release all the lanthanides from the chelates on the solid support, but only the newly-formed Eu and Sm chelates will be fluorescent. After measurement of Eu and Sm, DELFIA Enhancer is added which contains a new ligand for lanthanides. This ligand takes lanthanide ions from the chelate in Enhancement solution and forms a fluorescent chelate with Tb. When Tb is used in the assay, this sequential enhancement has to be performed for measurement.
Consequently, single-label DELFIA assays are performed with Eu-labeled reagents. Dual-label assays are performed with Eu and Sm or Tb. The choice is dictated by the required sensitivity and assay protocol. If the sensitivity of Sm is high enough for the assay, then it can be used together with Eu. However, higher sensitivity of Tb is sometimes needed, in which case the more complex sequential measurement has to be used. Of course, when an Eu/Sm pair is used, Eu is selected for the parameter requiring higher sensitivity. Triple-label assays are performed with Eu, Sm and Tb.
Microplate selection
Revvity has several microplates that display low fluorescence in Eu and Sm measurements. These plates include DELFIA clear and yellow plates, and OptiPlates™ (clear and black 96-well plates, and clear and white 384-well plates). The criteria for a good clear 96-well plate are that the background is less than 200 counts when measured empty using the DELFIA Europium measurement settings.
Assays that include Tb greatly benefit from the use of DELFIA yellow plates. These plates give lower background compared to clear plates, without any sacrifice in signal.
White CulturPlates™ or TC-treated Viewplates™ are recommended for adherent cell assays.
Incubation
Each assay requires optimization, but in general, buffer optimization will be the same for all the lanthanides. We recommend a Tris-HCl buffered (pH 7.5-8) assay buffer containing BSA (0.02-0.5%), detergent (e.g. 0.001-0.01% Tween 20 or 40), saline, possible immunological blockers, and a low concentration of chelating agent (20 µM EDTA or DTPA). We offer DELFIA Assay buffer (50 mL, catalog number 1244-106; 250 mL, catalog number 1244-111), which contains all the above components at optimized concentrations for most assays. Please note that DELFIA Assay buffer doesn’t contain immunological blockers.
Suitable concentrations for labeled reagents in sandwich multi-label assays are 200-500 ng/mL for Eu- and Tb-labeled antibodies, and 500-1500 ng/mL for Sm-labeled antibodies.
Measurement
Measurement of lanthanides is described under General Tips. Measurement in 96-well plates is started by dispensing (e.g. with Eppendorf Multipipette using a dedicated tip) the Enhancement solution to the wells (100 or 200 µL per well). Then the plate is shaken on a DELFIA Plateshake for 5 minutes. Other shakers can be optimized easily: shaking for 5 minutes, measurement, shaking for 5 minutes, measurement, etc. Eu and Sm signals are measured. Next DELFIA Enhancer is added (if Tb-labeled reagents have been used). When 200 µL Enhancement solution has been dispensed per well, then you would use 50 µL DELFIA Enhancer. After shaking for 1-2 minutes as above, you will measure the Tb signal.
The Revvity VICTOR™ Multilabel Plate Reader has a Eu-Sm dual label protocol. The Europium protocol measures pure Eu signal (615 nm, delay 400 µs, window 400 µs). However, because Eu has a small emission peak at Sm channel (642 nm), contribution of Eu to Sm measurement has to be taken into account. The VICTOR does this after Eu-Sm normalization (which can be found in Tools). You will need the type of plate you will be using for your assay, Enhancement solution and 1 nM Eu standard in Enhancement solution (50 mL, catalog number B119-100). Then follow instructions. This normalization is plate-specific and is performed only once. Sm in Eu window measurement (642 nm, delay 400 µs, window 400 µs) is used to calculate the contribution of Eu to Sm signal. The actual Sm measurement (642 nm, delay 50 µs, window 100 µs) gives automatically corrected Sm counts (if normalization has been performed; if not, the instrument gives just Eu counts even though it measured all three parameters).
Cell-based multiplexing
When performing cellular assays, in particular with whole cells (adherent), there are a couple of technical issues to be addressed. First, the buffer should contain chelating agents to prevent any free lanthanides from accumulating into membranes. Our buffer contains 20 µM of DTPA, but it could be replaced with something like 100 µM EDTA. Replacing the N1 chelate with a DTPA chelate could also help in controlling the background. The second technical issue relates to washing, which should be efficient without destroying your cells. Last, when choosing the labels, one should understand that Sm gives less signal (and may not give the same sensitivity, depending on background) compared to Europium and Terbium. Scientists who need two labels and cannot compromise sensitivity generally choose Eu and Tb. However, this combination of lanthanides requires the addition of Enhancer before Tb measurement.
Brochures, posters and other resources
- DELFIA multiplexing brochure - contains excitation and emission wavelengths for Europium, Terbium, Samarium, and Dysprosium, as well as citations and other tips
- Poster for multiplexed cell-based kinase assay - detection of phospho-ERK1 and phospho-ERK2 in fixed cells using Europium- and Samarium-labeled antibodies
- Poster for multiplexed cell proliferation and DNA fragmentation assay using Europium- and Samarium-labeled reagents
Citations
View a brief listing of DELFIA multiplexing citations.
Custom labeling and assay development services
Revvity offers custom labeling services as well as custom assay development. If you are interested in having your biomolecule custom-labeled, or in custom assay development, please contact our custom teams:
Custom Labeling and Conjugation Services
Custom Assay Development Services
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























