Overview
DELFIA™ kinase assays are wash-based assays. DELFIA can be used to detect the phosphorylation of tagged or untagged peptide or protein substrates in vitro (in biochemical assays with purified reagents), in cellular lysates (using cellular lysates as the source of kinase or detecting the phosphorylation event of a substrate within a cell), or in fixed cells.
In a typical biochemical DELFIA kinase assay, a peptide substrate or protein substrate is used. The substrate is usually biotinylated or otherwise tagged so that it can bind to a pre-coated plate. Untagged substrates can also be used, as long as you have an antibody that will bind the substrate without sterically hindering the binding of the anti-phospho antibody.
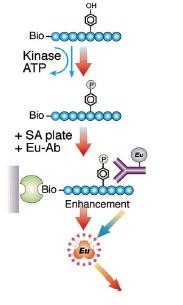
In the first step of a biochemical kinase assay, the kinase is incubated with substrate and ATP. In the second step of the assay, the reaction is added to an appropriate microplate that will bind to the substrate. A Europium-labeled anti-phospho antibody is added. After wash steps, DELFIA Enhancement solution is added for detection of the DELFIA Europium.

Flow diagram for DELFIA kinase assay using a biotinylated peptide substrate and a streptavidin-coated plate.
If you have sandwiching antibodies (one that will bind your "total" protein, and another that specifically recognizes the phosphorylated site), you can use an anti-species coated plate or perform a direct coating of your "total" antibody onto an uncoated DELFIA plate.
The assay can be adapted to work in more-complex matrices such as cell lysates (either using a transfected or transformed cell line expressing the tagged substrate) or by detecting the presence of an endogenous, phosphorylated substrate using the sandwiching antibody technique. You could also perform a fixed-cell assay (see application note and poster below).
What do I need to run this assay?
For a biochemical assay:
Required reagents available from Revvity:
- DELFIA Europium-labeled anti-phospho antibody (or Eu-labeled secondary anti-species antibody with an unlabeled primary anti-phospho antibody)
- DELFIA streptavidin-coated microplate (or any plate modified to bind your substrate)
- DELFIA wash buffer
- DELFIA assay buffer
- Seal™-A adhesive plate seal (do not use adhesive seals once Enhancement solution has been added to the wells)
- DELFIA Enhancement solution
Required reagents available from various suppliers:
- Kinase
- Kinase buffer
- ATP
- EDTA (to s the kinase reaction)
- Biotinylated substrate (or otherwise tagged substrate with the appropriate capture plate, or untagged substrate if antibody is available to capture the substrate to a microplate)
Instrumentation/equipment:
- A TRF-capable plate reader
- Optional: Plate shaker (we recommend our DELFIA Plateshake)
- Optional: Plate washer (we recommend our DELFIA Platewash)
For cell-based assays, we recommend either a sandwiching antibody assay design (using one antibody that will recognize your substrate in general, and one antibody that will recognize the phosphorylated site on your substrate) or a fixed-cell assay. Please see our page on DELFIA immunoassays for helpful information.
Products and catalog numbers
View a listing of DELFIA products and catalog numbers.
Application notes and posters
- Application note for detecting phosphorylation of p44/42 MAPK in fixed cells
- Poster for a cell-based multiplexing kinase assay (using fixed cells)
- NIH assay guidance (an external assay guidance website provided by the National Institutes of Health - describes Km, Vmax, IC50, etc. for enzymatic reactions)
Citations
View a brief list of DELFIA kinase citations.
DELFIA kinase troubleshooting
|
Problem |
Cause |
Solution |
|---|---|---|
|
No counts or very low counts |
Low enzyme activity |
Check the enzyme activity. |
|
Check that the enzyme works with substrate. |
||
|
Use of adhesive tape during enhancement step |
Adhesive tape must not be used during the enhancement step. |
|
|
Kinase reaction buffer |
The kinase reaction buffer should contain a low concentration (0.005-0.05%) detergent (e.g., Brig-35, NP40, CHAPS etc.) to prevent binding of the reaction components to the reaction vial/well. |
|
|
High background |
Presence of aggregated immunoglobulins |
After storage, filter the Eu-labeled anti-phosphotyrosine antibody through a 0.2 µm membrane. |
|
Inadequate washing prior to measurement |
After incubation with Eu-labeled antibody, 4-6 washing cycles with DELFIA Platewash is adequate. When washing the plates, ensure that each well is filled up completely to the . After washing, check that the wells are dry. With other plate washers, the number of wash cycles will need to be optimized (the required number of wash cycles could be higher). |
|
|
Poor reproducibility |
Inadequate washing prior to measurement |
See above. |
|
Too short incubation with Enhancement solution |
Incubate for least 5-10 minutes on the shaker before measurement. For every shaker, check the time needed for maximal signal. |
|
|
Inadequate pipetting technique |
Check your pipetting technique. A dedicated Eppendorf Multipipette or Dispenser is recommended for dispensing Enhancement solution. |
|
|
Poor sensitivity |
|
For high background, see above. |
|
Optimize the concentration of the enzyme, substrate and ATP in the assay. |
Custom labeling and custom assay development at Revvity
Revvity offers custom labeling services as well as custom assay development.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























