Overview
The DELFIA™ cell cytotoxicity assay kit was designed to measure cell-mediated cell cytotoxicity. The assay has also been used for antibody-dependent cell-mediated cytotoxicity (ADCC). This is a non-radioactive alternative to the conventional Chromium-51 (51Cr) release assay, and works on the same principle as the radioactive assay. In the standard cell-mediated cytotoxicity assay format, you will have two types of cells: target cells and effector cells.
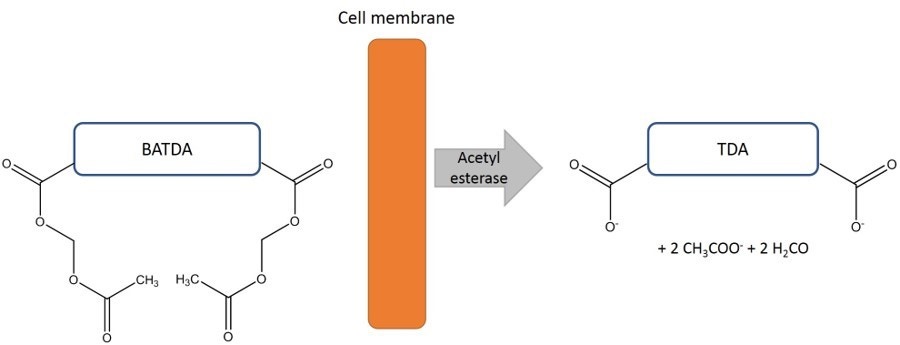
Target cells are first loaded with an acetoxymethyl ester of BATDA. The ligand penetrates the cell membrane quickly. Within the cell, the ester bonds are hydrolyzed to form a hydrophilic ligand (TDA), which no longer passes through the cell membrane.
- While cells are intact, TDA remains in the cell. When Europium solution is added to supernatant from a sample of intact cells, the Europium is unable to form a fluorescent chelate with TDA, as no TDA was released into the supernatant. Europium solution is not fluorescent in its unaltered state.
- If cells are lysed by an effector cell, TDA is released outside the cell into the supernatant. Upon addition of Europium solution to the supernatant, Europium can form a highly fluorescent and stable chelate with the released TDA (EuTDA). The measured fluorescence signal correlates directly with the amount of lysed cells in the cytotoxicity assay.

Figure 1. Principle of cell labeling. After BATDA ligand has penetrated the cell membrane, it is hydrolyzed to TDA by the acetyl esterases in the cell.
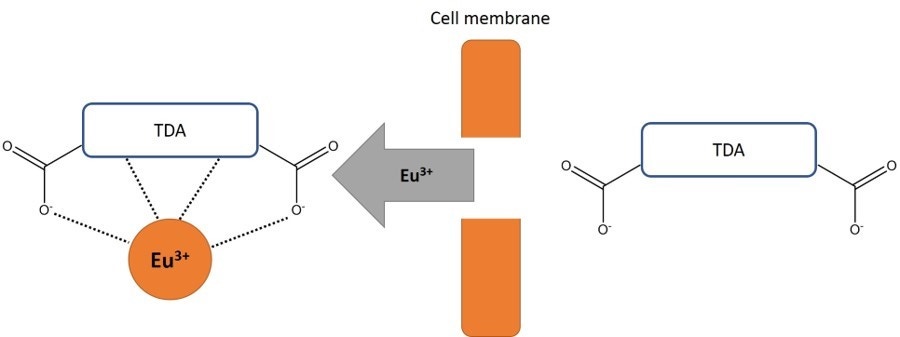
Figure 2. Detection of the released TDA. When effector cells are mixed with target cells, the target cells are lysed. After addition of Eu3+ solution to the sample, a fluorescent chelate is formed.
What do I need to run this assay?
Required reagents available from Revvity:
- DELFIA cell cytotoxicity assay kit (catalog number AD0116)
- Reagents for the assay are also available separately:ysis buffer (30 mL), #4005-0010
- Lysis buffer (30 mL), #4005-0010
- DELFIA Eu-Solution (200 mL), #C135-100
- DELFIA BATDA labeling reagent (50 µL), #C136-100
Required reagents available from other suppliers:
- V-bottom cell culture plates (we use Sigma #M9686-100EA or Revvity polypropylene V-bottom plate #6008290 for suspension cells)
- PBS or DPBS
- Cells
- Culture media
- *The DELFIA Cell Cytotoxicity kit (#AD0116) comes with a clear, stripwell 96-well plate for the detection step. If you are buying reagents separately, you can use any clear or white polystyrene plate that is suitable for time-resolved fluorescence (e.g., white OptiPlate #6005290 or white 1/2 AreaPlate #6002290)
Instrumentation/equipment:
- A TRF-capable plate reader
- Optional: Plate shaker (we recommend our DELFIA Plateshake)
Protocol-in-brief
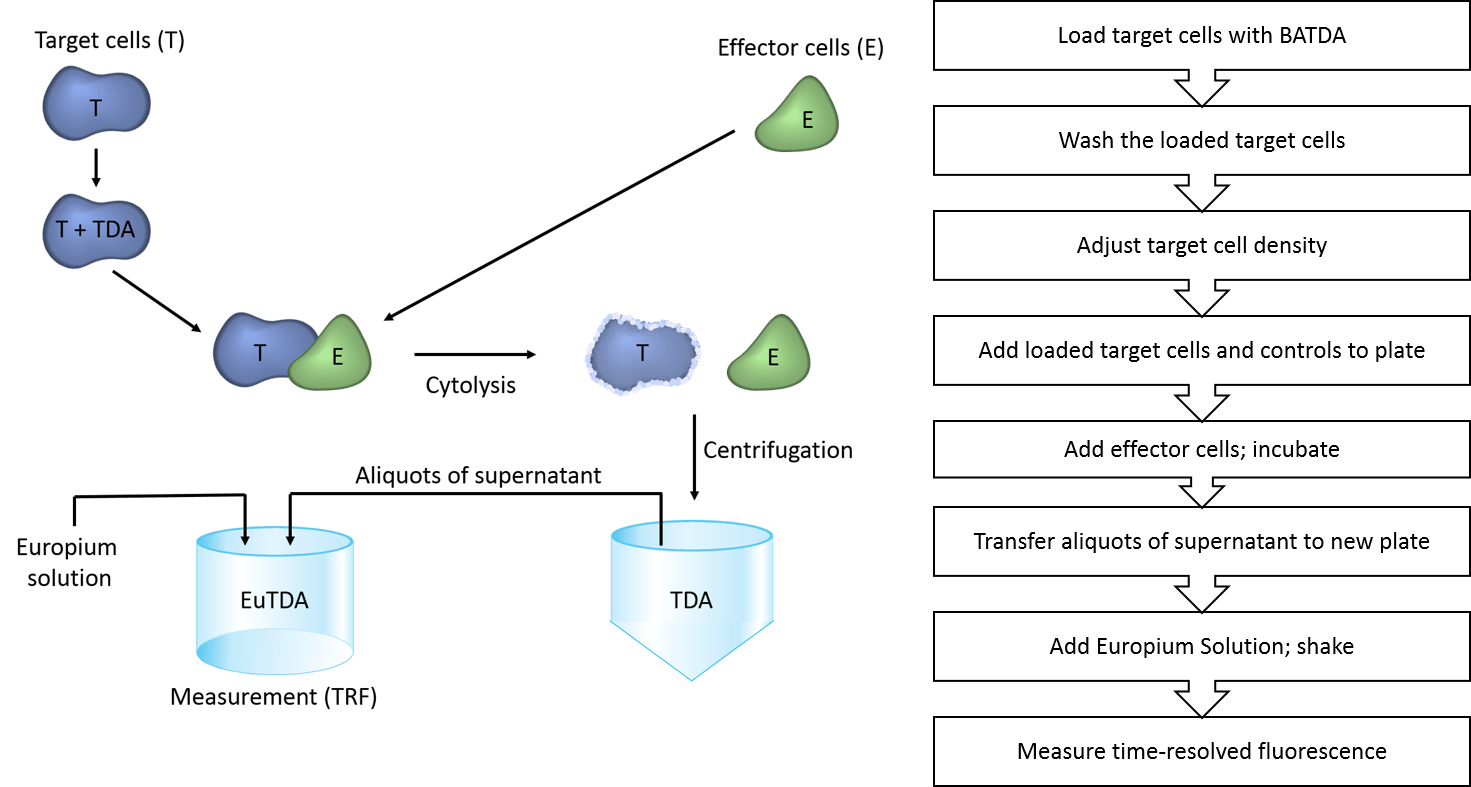
The protocol used in DELFIA cell cytotoxicity assays is summarized in Figure 3. Please refer to the manual for more-detailed information.
Definitions/Samples
Background (media without cells): An aliquot is taken immediately from the diluted, loaded target cell suspension; this is then centrifuged to pellet the cells. 100 µL of supernatant is pipetted into wells and 100 µL of medium is added.
Spontaneous release (target cells without effector cells): Loaded target cells (100 µL) are incubated with 100 µL of medium, instead of with effector cells. The spontaneous release is monitored over time, and samples for spontaneous release are taken at the same time points as when the assay samples are taken.
Maximum release (lysed target cells): Loaded target cells (100 µL) are incubated with 100 µL of medium supplemented with 10 µL of DELFIA Lysis buffer.
Probenecid*: An inhibitor of the multidrug resistance transporter (MDR); needed for some cell types to prevent spontaneous release. Probenecid should be prepared fresh every day at a stock concentration of 250 mM, and used at a working concentration of 2.5 mM.
- Solubilize 710 mg of probenecid in 5 mL of 1.0 N NaOH
- Add 5 mL of PBS 20 mM HEPES (the concentration of probenecid is now 250 mM)
- Mix and transfer back to PBS 20 mM HEPES to make a 2.5 mM working concentration. Measure the pH to make sure it was not affected by the addition of probenecid.
* Mitomycin C and sulfinpyrazone can also be used.
Application notes, example of data, and other resources
- DELFIA cell cytotoxicity assay tech note: This application note describes an assay using K562 target cells with PBL or NK effector cells. A comparison with the Cr-51 release assay is shown. The assay was performed in a 384-well format without significantly affecting the results, and the effect of freezing samples was studied. There is also a troubleshooting section.
- DELFIA cell cytotoxicity assay application note: This application note has protocols-in-brief, and contains data for spontaneous and maximum release in P815 and CHO cells. Additional troubleshooting tips are included.
- Sample protocol and data in Excel format from a VICTOR™ plate reader, for an assay using K562 target cells and peripheral blood mononuclear cells (PBMs).
Citations
View a brief list of DELFIA cell cytotoxicity citations.
Troubleshooting
|
Problem |
Cause |
Solution |
|---|---|---|
|
Low recovery of loaded cells |
Poor conditions |
Check the composition and pH of the buffers and solutions. |
|
Shorten the loading time. |
||
|
Be careful when washing the cells. If necessary, reduce the number of washes to three. |
||
|
Use the culture medium supplemented with serum throughout the assay. |
||
|
Perform the cell loading under optimal culturing conditions. |
||
|
High background |
Inadequate Washing |
Wash the loaded cells 5-6 times before use, carefully but quickly. Centrifuge for a maximum of 5 minutes at a speed suitable for live cells. |
|
Solutions or pipettes |
Avoid carryover from one wash to the following wash. |
|
|
Check that your solutions are not contaminated with BATDA. |
||
|
Check for culture medium interference. |
||
|
Low label incorporation/poor sensitivity |
Cell line difficult to label |
Increase the label concentration and/or the labeling temperature. |
|
Cells are in poor conditions |
Try to use Pluronic F-127, 0.02% (w/w) during loading time. |
|
|
Optimize the loading temperature. |
||
|
Check the pH of the loading buffer. |
||
|
Low maximum release |
Quench of signal |
SDS quenches fluorescence, so use the lysis buffer from the kit or Triton X-100. |
|
High spontaneous release |
Dependent on cell line |
This test is suitable for short-term assays (<4 h). Long incubation times increase the spontaneously release. Withdraw supernatant samples after only a few minutes of incubating the target cells with effector cells, and monitor the release time. |
|
Note that this assay is typically faster than 51Cr release. |
||
|
Decrease the loading time. |
||
|
Avoid leaving the cells waiting after loading and washing. |
||
|
Try adding 1-10 mM probenecid or 1-10 µg/mL mitomycin C to the wash solution and to the medium used for diluting the cells after washing. Note: Probenecid is not soluble in the wash buffer or the medium at these concentrations. A 200X stock solution is first dissolved in 1 M NaOH, which is then diluted 2-fold in the wash buffer (1X PBS supplemented with 20 mM HEPES). The 100X probenecid stock solution is then diluted 100-fold in the wash buffer and the medium. |
Tips
- Please note that the incubation times for the DELFIA cytotoxicity assay are usually shorter than for a Cr51-release assay. Increased incubation times (>4 hours) will usually result in high spontaneous lysis.
- The first time you run the assay, we recommend that you remove 20 µL supernatant samples from your plate at various time points (15 minutes, 30 minutes, 1 hour, 1.5 hours, 2 hours, etc.). You do not need to set up a well for each time point; you can simply remove a portion of the supernatant from your assay plate and transfer it to another plate, then add Europium solution to the aliquots and read. You do not need to spin the plates containing the cells down if you are careful not to disturb your cells when pipetting.
- One of the troubleshooting tips in the application note above suggests "conditioning cells after labeling" if you have high spontaneous release. This refers to putting the cells in complete culture media and into the cell incubator for a short time to feed them and make them warm.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























