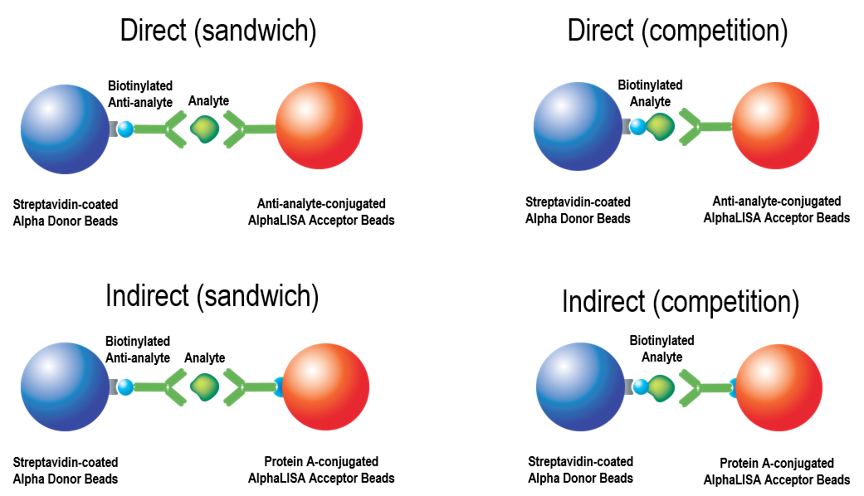
Overview
The information on these pages offers guidance for the design and development of any Alpha assay. Please note that there are two sections that link to individual pages containing detailed information: one for those who will be developing an immunoassay specifically, and the other for those who will be working with tagged reagents as one of their assay components. Further below, you will find a section that discusses how to conjugate your own reagent to a bead.
Immunoassays
Detailed information on immunoassay design and development using the Alpha technology. Many assays (analyte detection, antibody quantitation/characterization, post-translational modifications, kinase assays, protease assays, etc.) can be designed as immunoassays. Existing ELISAs can be converted to a no-wash AlphaLISA™ assay. Assays can be used for screening or quantitation of an analyte (by running samples against a standard curve created with a recombinant analyte). There are two major formats: a sandwiching antibody assay and a competition (displacement) assay. Each format can be performed using directly conjugated beads (one antibody directly conjugated to an AlphaLISA Acceptor bead), or via an indirect method of antibody-bead association (Figure 1).
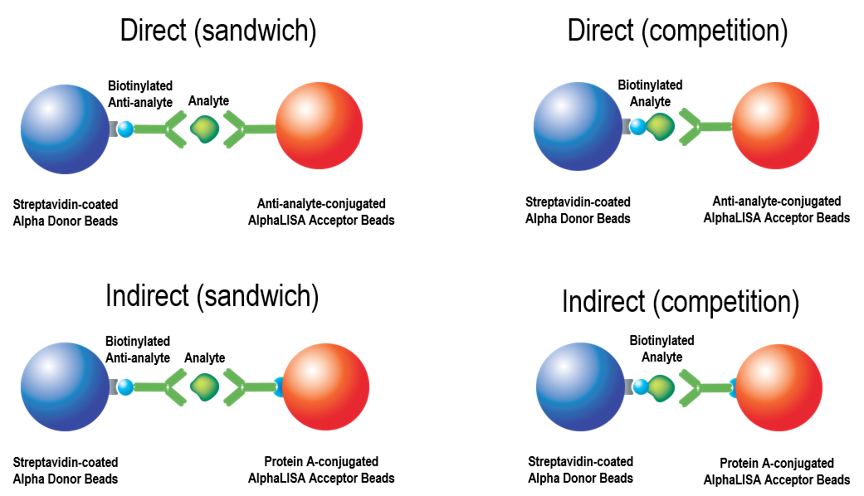
Figure 1. Sandwiching and competition assay formats in Alpha, and direct vs. indirect antibody association methods.
Assays That Use Tagged Reagents
Detailed information on how to design and develop Alpha assays that use tagged reagents. The use of tagged proteins, peptides, and other reagents (6xHis, GST, c-myc, HA, digoxigenin, etc.) can be particularly useful when studying biomolecular interactions or enzymatic activity with recombinant or synthetic substrates (kinase assays, protease assays, helicase assays, etc.). A tagged reagent with an appropriate anti-tag bead or affinity bead can be used in place of a target-specific antibody in your assay design (Figure 2).
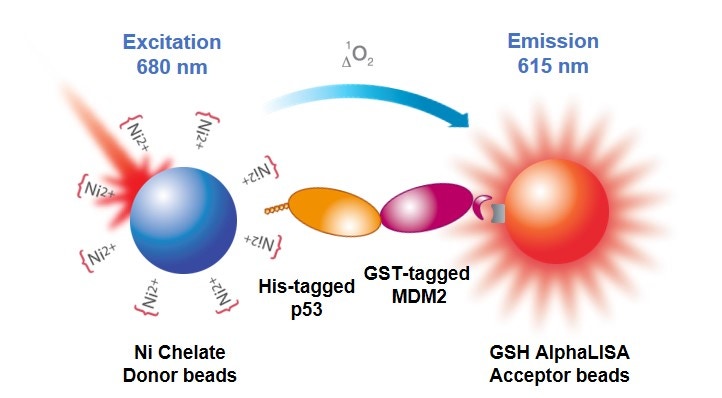
Figure 2. Alpha assay that uses tagged reagents (His-p53, GST-MDM2) in a protein-protein interaction assay. The His-tagged p53 associates with a nickel chelate bead, and the GST-tagged protein associates with a glutathione-coated bead.
Bead Conjugation
Detailed information, tips, and FAQs for bead conjugation. You can use our unconjugated beads to directly conjugate a peptide, protein, or antibody of interest directly to an Alpha Donor, AlphaScreen Acceptor, or AlphaLISA Acceptor bead. Our unconjugated beads can be used in a reductive amination reaction to link your reagent via free amines (lysines, N-termini) as shown in Figure 3.
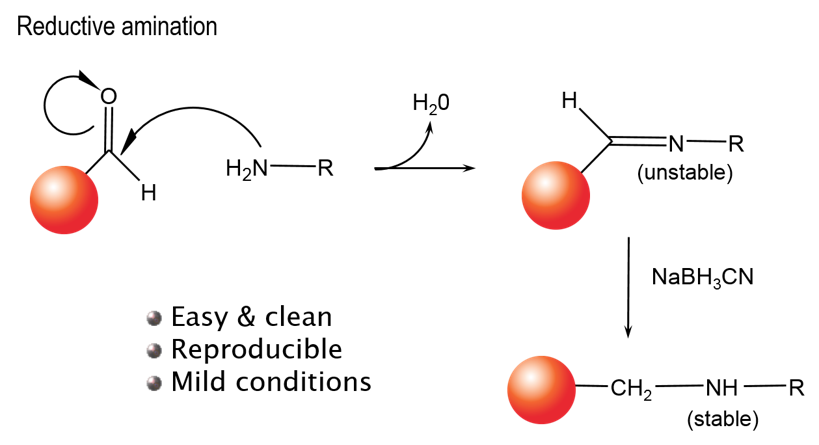
Figure 3. Reductive amination conjugating reaction.
Working Range of Binding Affinities
AlphaLISA assays have a very broad working range in terms of binding affinity, as demonstrated in Figure 4. This makes the assays particularly well-suited for antibody-based assays and for protein-protein interaction assays, where binding affinities are typically much lower.
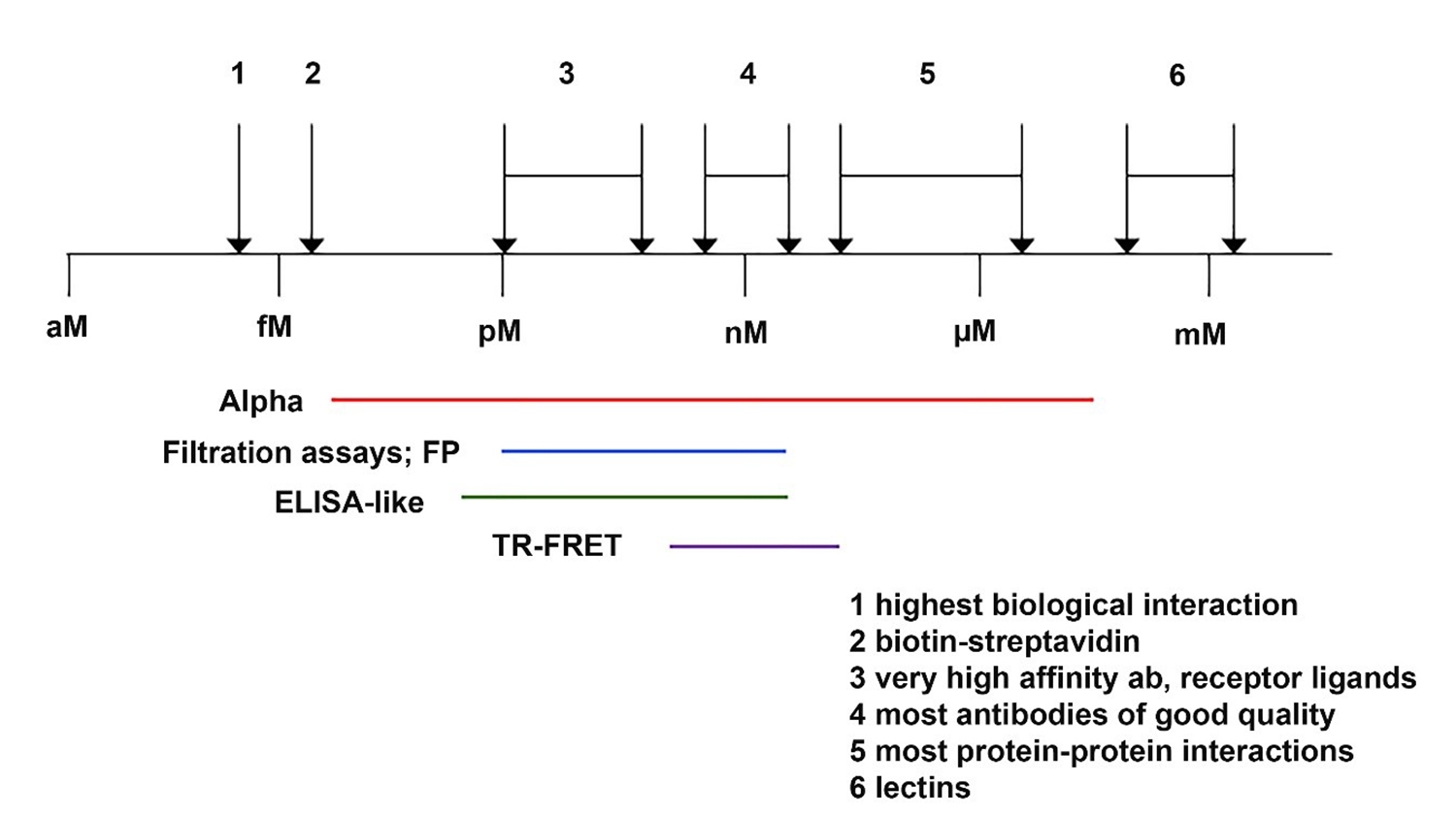
Figure 4. Binding affinities for various biomolecular interactions. Key: 1 = highest affinity biological interaction, 2 = biotin-streptavidin interaction, 3 = very high affinity antibody or receptor ligands, 4 = most antibodies of good quality, 5 = most protein-protein interactions, 6 = lectins.
Available Choices of Donor and Acceptor Beads
Bead selection is an important aspect of assay design. Bead selection can impact assay performance, and in some cases, can mean the difference between a successful assay and an unworkable assay. An understanding of the various ways bead choice can affect your assay may be helpful when you are planning your experiments.
Theoretical Bead Binding Capacities
Relative capacities of various beads (beads used at 20 μg/mL final concentration in assay).
|
Bead Coating |
Used to Bind/Capture |
Molecule and Partner Bead Used to Determine Theoretical Binding Capacity |
Theoretical Binding Capacity1(Provided for Relative Comparison) |
|---|---|---|---|
|
Streptavidin |
Biotinylated peptides, proteins, oligos, sugars, small molecules, etc. |
Biotinylated peptide |
30 nM |
|
Strep-Tactin® |
Strep-Tag® II and One-STrEP tagged proteins/peptides |
One STrEP-azurin-6X His with nickel chelate beads (100 nM) |
|
|
Anti-GST antibody |
GST-fusion proteins and peptides |
Biotinylated GST with streptavidin bead |
3 nM |
|
Anti-6X His antibody |
His-tagged proteins and peptides |
6X His-GST with glutathione bead |
100 nM |
|
Anti-FLAG antibody |
FLAG-tagged proteins and peptides |
Biotinylated FLAG with streptavidin bead |
100 nM |
|
Anti-maltose binding protein (MBP) antibody |
MBP-tagged proteins |
Biotinylated MBP with streptavidin bead |
1 nM |
|
Anti-HA antibody |
Hemagluttinin-tagged proteins and peptides |
Biotinylated-PEG-HA with streptavidin bead |
10 nM |
|
Anti-c-myc antibody |
c-myc-tagged proteins and peptides |
Biotinylated c-myc with streptavidin bead |
100 nM |
|
Anti-DIG antibody |
Digoxigenin labeled proteins, peptides oligos, etc. |
Biotinylated digoxigenin with streptavidin bead |
1 nM |
|
Anti-FITC antibody |
FITC or fluorescein-labeled proteins, peptides, oligos, sugars, small molecules, etc. |
Biotinylated-ERE-FITC with streptavidin bead |
>1 nM |
|
Anti-V5 antibody |
V5-tagged proteins/targets |
Biotin-Chromalink V5 (14 aa) with streptavidin bead |
~3 nM |
|
Anti-GFP antibody |
GFP-tagged (green fluorescent protein-tagged) proteins and peptides |
Biotinylated GFP with streptavidin beads |
3 nM |
|
Glutathione (GSH) |
GST-fusion proteins and peptides |
6X His-tagged GST with nickel chelate bead |
300 nM -1 μM |
|
Nickel chelate (Ni2+) |
His-tagged proteins and peptides |
6X His-tagged GST with glutathione bead |
300 nM -1 μM |
|
Protein A |
Antibodies2 |
Biotinylated rabbit IgG with streptavidin bead |
3 nM (antibody3) |
|
Protein G |
Antibodies4 |
Biotinylated rabbit IgG with streptavidin bead |
1 nM (antibody3) |
|
Protein L |
Antibodies5 |
Biotinylated human IgG (kappa) with streptavidin bead |
1 nM (antibody3) |
|
Anti-human IgG |
Fc portion of human IgG antibodies |
Biotinylated human IgG with streptavidin bead |
3 nM (antibody3) |
|
Anti-rabbit IgG |
Fc portion of rabbit IgG antibodies |
Biotinylated rabbit IgG with streptavidin bead |
1 nM (antibody3) |
|
Anti-mouse IgG |
Fc portion of mouse IgG antibodies |
Biotinylated mouse IgG with streptavidin bead |
3 nM (antibody3) |
|
Anti-mouse IgM |
Mouse IgM immunoglobulins |
Biotin-mouse IgM with streptavidin bead |
0.3 nM (antibody3) |
|
Anti-rat IgG |
Fc portion of rat IgG antibodies |
Biotinylated rat IgG with streptavidin bead |
1 nM (antibody3) |
|
Anti-sheep IgG |
Fc portion of sheep IgG antibodies |
Biotinylated sheep IgG with streptavidin beads |
1 nM (antibody3) |
|
Anti-goat IgG |
Fc portion of goat IgG antibodies |
Biotinylated goat IgG with streptavidin bead |
3 nM (antibody3) |
|
Anti-chicken IgY |
Fc fragment of chicken IgY immunoglobulins |
Biotinylated chicken IgY with streptavidin beads |
0.3 nM (antibody3) |
- The numbers provided are examples given to compare relative bead capacities, and are derived from probe titration experiments, as shown in the QC data on the product tech data sheets. The actual bead capacity for your bead system should be determined empirically for a given assay. The bead capacities given in this table are influenced by partner bead and the size of the probe used in these assays. The actual bead capacity will be influenced by size of the protein associating with the bead, as well as affinity of the protein (or antibody) for the bead.
- Protein A interacts strongly with particular subclasses of antibodies, including human IgG1, IgG2, and IgG4; mouse IgG2A and IgG2B; and rabbit, human and mouse total IgG. Protein A also has weaker affinity for other antibody subclasses. The affinity depends on the isotype and species of the antibody associating with the bead. Pierce has a nice chart that may be helpful.
- The bead capacity for these products refers to the capacity for antibody. The amount of protein you will then be able to bind to the antibody on the bead will be dependent on the affinity of your antibody for your protein and the size of your protein, and may range anywhere from 1 pM to 1 μM.
- Protein G binds to all subclasses of human IgG and mouse IgG. In addition, it binds to rat, goat, sheep, guinea pig, rabbit, cow, pig and horse antibodies. Pierce has a nice chart that may be helpful.
- Protein L binds efficiently total human IgG, IgM, IgA, IgE, IgD, mouse IgG and rat IgG. It binds only poorly mouse IgM and rabbit IgM and it does not bind human IgG (lambda light chain), rabbit, sheep, goat and bovine IgG and rat IgM. Pierce has a nice chart that may be helpful.
Buffer Selection
Visit Buffer selection for Alpha assays for help in choosing a suitable buffer for your assay. This page describes the various Alpha immunoassay buffers supplied by Revvity and will help you avoid interfering substances in buffers of your own design.
Working With Serum Samples
When analyzing samples in serum, it is best to prepare the standard curve in a sample matrix as similar as possible to the actual samples. This will help ensure that the assay accuracy is as high as possible.
Analyte-Depleted Serum Standard Curve
For serum samples, the best option is to prepare the standard curve in analyte-depleted serum.
FBS Standard Curve
When analyte-depleted serum is not conveniently available, the analyte standard curve can be prepared in 100% FBS (fetal bovine serum). The same lot of FBS should be used for all asays in a given study. To ensure high accuracy (percent recovery), it may be necessary to dilute the serum sample somewhat (perhaps 2- or 4-fold) in FBS.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























