Overview
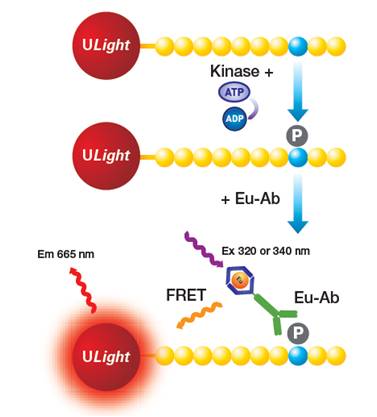
The LANCE™ Ultra kinase assay uses a ULight™-labeled peptide substrate and an appropriate Europium-labeled anti-phospho antibody (Figure 1). When the substrate becomes phosphorylated by your kinase, the phosphorylated site on the substrate is recognized by the Europium-labeled anti-phospho antibody. Upon excitation of the Europium donor fluorophore at 320 or 340 nm, energy is transferred to the ULight acceptor dye on the substrate, resulting in the emission of light at 665 nm. The intensity of light emission is proportional to the level of ULight peptide phosphorylation. One distinct advantage of the LANCE Ultra kinase assay is that it is usually a two-component assay (ULight substrate + Eu-anti-phospho antibody), which makes assay development easier and faster.

Figure 1. LANCE Ultra kinase assay using a ULight-labeled peptide substrate and a Europium-labeled anti-phospho antibody.
The assay is usually performed in 96-well, 384-well, or 1536-well plates. The assay is performed in two steps:
- The first step is the kinase reaction. In this step, your kinase is mixed with the ULight-substrate, ATP, and any inhibitors or molecules being tested (if relevant), in a suitable buffer. The reaction is incubated for a period of time, at an appropriate temperature for your kinase (usually at room temperature).
- The second step of the assay is the detection step. After addition of EDTA to your kinase reaction, the appropriate LANCE Europium-anti-phospho specific antibody (donor fluorophore) is added to the reaction in LANCE Detection buffer. The mixture is allowed to incubate for a period of time to allow binding of the antibody to the phosphorylated site before the plate is read.
LANCE Ultra kinase substrates
Revvity provides a variety of generic ULight-labeled peptide substrates for both serine/threonine and tyrosine kinases. We also provide an extensive list of validated substrates for various kinases. Core motifs for each substrate are shown in our substrate guide, found here on page 11 for serine/threonine kinase substrates, or here on page 16 for tyrosine kinase substrates. In addition to our standard catalog products, we can custom-label your own peptide substrate sequence with ULight if you desire. It is also possible for you to label your own antibody with LANCE Europium, or to have this performed as a custom labeling.
For serine/threonine kinases, we offer many ULight peptide substrates. Please refer to our kinase substrate selector guide. If your kinase is not on our list, our Serine/Threonine KinaSelect™ kit (catalog number TRF0300-C) can be used to test various substrates. This kit provides five ULight peptide substrates, along with the appropriate anti-phospho specific antibodies and our LANCE Detection buffer. The five ULight substrates provided in this kit have been shown to be suitable for over 80% of a panel of 184 kinases tested. The advantage of our Ser/Thr KinaSelect kit is that it provides smaller trial size quantities of five ULight substrates, five Eu-anti-phospho antibodies, and our LANCE detection buffer, all in one kit. Please note that ULight substrates are named for the protein/kinase from which the peptide sequence was derived, not for the kinase that the substrate works with.
For tyrosine kinases, in addition to traditional generic random polymer ULight-poly GT and ULight-poly GAT substrates, we provide several other ULight-labeled tyrosine kinase substrates. Please refer to our kinase substrate selection guide. If your tyrosine kinase is not on this tool, you could try the ULight-TK substrate. Our newest tyrosine kinase substrate, ULight-TK is a 28-amino acid peptide that has been shown to be an improved substrate for 85% of cylasmic and receptor tyrosine kinases tested. In addition to being offered as a stand-alone peptide substrate, ULight-TK is also offered as a TK KinaSelect kit (catalog number TRF0301-D). The advantage of our TK KinaSelect kit is that it provides ULight-TK substrate, Eu-anti-phospho antibody, and our LANCE detection buffer, all in one kit. You can also check the literature to see if poly-GT or poly-GAT have been used with your kinase of interest.
What do I need to run this assay?
Required reagents available from Revvity:
- ULight peptide substrate (appropriate for your kinase; refer to the Kinase Substrate Selection Guides in the above section)
- Europium-labeled anti-phospho antibody (corresponding to the ULight substrate, if working with Ser/Thr kinases)
- 10X LANCE Detection buffer (#CR97-100)
- Microplate (We recommend white 384-well OptiPlate™ or ProxiPlate™ microplates.)
- Seal™-A adhesive plate seal (#6050195, #6005250)
Required reagents available from other suppliers (see where Revvity R&D gets these reagents):
- Kinase
- Kinase buffer: Basic kinase buffer - 50 mM HEPES pH 7.5, 1 mM EGTA, 10 mM MgCl2, 2 mM DTT, and 0.01% Tween 20). Add any essential kinase supplements (e.g., MnCl2, CaCl2, calmodulin, cGMP, lipids etc.) as appropriate for your kinase, if needed.
- EDTA
- ATP
- UltraPure water
Instruments/equipment:
- A TRF-capable plate reader (We recommend the Revvity EnVision™ or VICTOR™ Plate Reader.)
Products and catalog numbers
View a listing of our ULight-dye labeled peptide substrates and Europium antibodies for kinase assays.
Protocol-in-brief
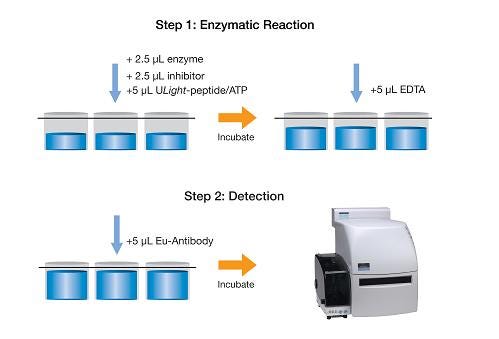
LANCE Ultra kinase assay workflow
Protocol 1: This is the suggested protocol for substrate selection, enzyme titration/time course experiments, and ATP titration optimization steps. This protocol allows you to easily omit ATP from your reaction as a control.
Stock solutions (for example, for "2X kinase" - if you want a final 1 nM kinase concentration in your reaction, make a 2 nM kinase stock):
- 2X kinase (prepared in kinase buffer)
- 4X ATP stock (prepared in kinase buffer)
- 4X ULight substrate stock (prepared in kinase buffer)
- 4X EDTA (prepared in 1X LANCE Detection buffer)
- 4X Europium-antibody (prepared in 1X LANCE Detection buffer)
Step 1: Kinase reaction (10 µL)
- 5 µL kinase
- 2.5 µL ULight substrate
- 2.5 µL ATP
Step 2: Detection (brings final volume in well to 20 µL)
- 5 µL EDTA
- 5 µL Europium-anti-phospho antibody
Protocol 2: This is the suggested protocol for inhibition curves and screening libraries in 384-well format. This protocol accounts for the addition of inhibitor or small molecule libraries to your assay.
Stock solutions (for example, for 2X ATP/ULight, if you want a final concentration of 100 µM ATP and 50 nM ULight substrate in your reaction, you will make a 200 µM ATP/100 nM ULight substrate stock solution):
- 4X kinase (prepared in kinase buffer)
- 2X ATP/ULight substrate stock (prepared in kinase buffer)
- 4X inhibitor stock (prepared in 2% DMSO or appropriate buffer)
- 4X EDTA (prepared in 1X LANCE Detection buffer)
- 4X Europium antibody (prepared in 1X LANCE Detection buffer)
Step 1: Kinase reaction (10 µL)
- 2.5 µL kinase
- 5 µL ULight substrate/ATP mix
- 2.5 µL buffer or inhibitor
Step 2: Detection (brings final volume in well to 20 µL)
- 5 µL EDTA
- 5 µL Europium-anti-phospho antibody
Assay optimization
1.Substrate identification/selection (if applicable)
2. Enzyme titration vs. time-course kinetics
3. ATP titration
4. Inhibition curve
5. Z' factor determination, if desired
Application notes, guides and other resources
A. Serine/Threonine kinases
- The technical data sheet and tech note specific for your ULight substrate - contain protocol information; these can be found on the product web page, under the Product Literature tab.
- Application note for a Ser/Thr kinase (describes in detail each assay development step for Erk1 kinase with ULight-MBP)
- Ser/Thr KinaSelect manual (describes substrate selection and assay development for Ser/Thr kinases, also includes a troubleshooting section and suggested list of suppliers for buffer components)
- Latest poster for serine/threonine kinases
- NIH assay guidance (an external assay guidance website provided by the National Institutes of Health - describes Km, Vmax, IC50, etc. for enzymatic reactions)
- Ser/Thr Kinase Substrate Selection Guide - contains recommended substrates for validated kinases
B. Tyrosine kinases
- The technical data sheet and tech note specific for your ULight substrate - contain protocol information; these can be found on the product web page, under the Product Literature tab.
- Latest poster for tyrosine kinases
- NIH assay guidance (an external assay guidance website provided by the National Institutes of Health - describes Km, Vmax, IC50, etc. for enzymatic reactions)
- Tyrosine Kinase Substrate Selection Guide - contains recommended substrates for validated kinases
Citations
View a brief list of citations describing LANCE kinase assays.
Tips and FAQs
- We suggest you use 6 mM final concentration EDTA (rather than 10 mM final concentration EDTA) to terminate your kinase reaction. This will increase the stability of your LANCE kinase signal. View our application note for more information.
- Keep in mind that generic anti-phosphotyrosine antibodies will recognize not only the phosphorylated site on your substrate, but also any phosphorylated tyrosines on your kinase. The more kinase in the reaction, the more antibody that can be depleted from your substrate because of the presence of phosphorylated tyrosine residues on your kinase. If you are working at tyrosine kinase concentrations that are too high, this may cause your results to appear as if you have a reverse enzyme titration effect (higher concentrations of kinase give lower signal).
- We recommend a final ULight peptide substrate concentration of 50 nM or 100 nM (as indicated on the tech data sheet for the product) in your kinase reaction when running LANCE Ultra kinase assays. Because this is a two-component assay and the substrate is directly labeled with fluorophore, increasing the amount of substrate will also increase your assay background. We do not recommend ULight-substrate concentrations higher than 200 nM.
-
If you would like to perform a substrate titration (for example, you are trying to determine the Km for substrate) and you are using a ULight-substrate, you will need to include controls with ULight substrate but no ATP at every concentration of ULight you test. This will allow you to correct for background. You will need to plot this data as signal minus background (S--B). This correction needs to be performed because the substrate you are titrating is directly-labeled with a fluorophore. Your background will increase with increasing concentrations of substrate.

Substrate titration experiment
Q. I'm studying a tyrosine kinase. Which anti-phosphotyrosine antibody should I use?
A. We have three generic anti-phosphotyrosine antibodies. Though any of these could be used with any ULight tyrosine kinase substrate, information in the Tyrosine kinase selection guide was generated using our PT66 antibody (catalog numbers AD0068, AD0069).
Q. Can I purchase pre-phosphorylated ULightsubstrate "standards"?
A. Please inquire with our custom services team (contact information below). We will need to know if you are using the phosphorylated standard for quantitation, or if you simply need a qualitative control.
Q. Can I miniaturize the assay?
A. Yes. Please refer to our LANCE Ultra kinase miniaturization poster for guidance.
Q. My signal decreases with increasing concentrations of kinase. What am I doing wrong?
A. If you are using a tyrosine kinase with one of our generic anti-phosphotyrosine antibodies (Figure 2), please be aware that the generic antibody can bind not only your phosphorylated substrate, but also any phosphorylated tyrosines on your kinase. We usually recommend you use a tyrosine kinase concentration of 1 nM or less. As you increase the amount of tyrosine kinase in your assay, you will be depleting the generic anti-phospho antibody. This will cause your data to appear to exhibit a reverse kinase titration effect. This is only a phenomenon for kinase assays that use generic anti-phospho antibodies.
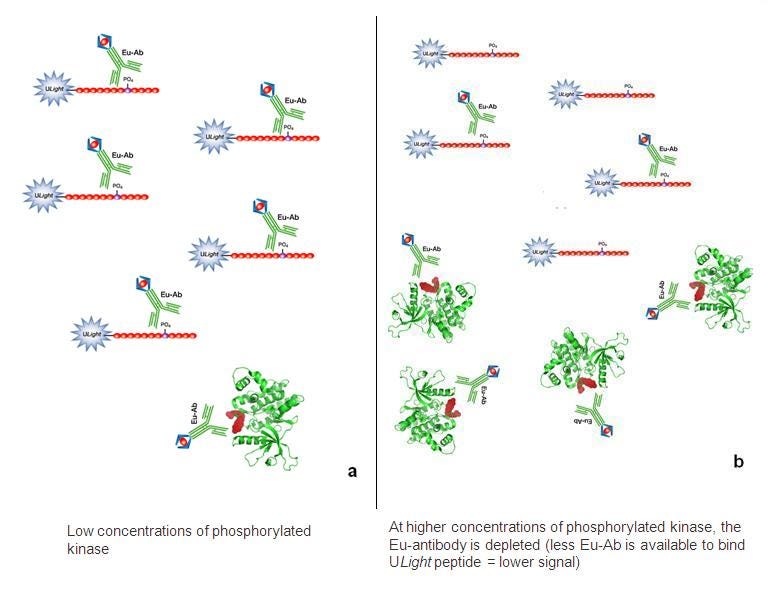
Figure 2. Generic anti-phosphotyrosine antibody depletion by increasing amounts of active tyrosine kinase.
Q. You have kinase concentrations in the LANCE Ultra tyrosine kinase selection guide. How were these concentrations determined?
A. Our R&D teams have run kinase titration curves for all validated tyrosine kinases (for example, see Figure 3). For these assays, we use phosphorylated ULight substrate and run a kinase titration curve (no ATP - the substrate is already phosphorylated). The concentrations listed are the maximal concentration that could be used without antibody depletion affecting the assay signal. You should still titrate your kinase and determine the optimal enzyme concentration for your needs. The concentration you choose might be much less than the kinase concentration listed in the selection guide.
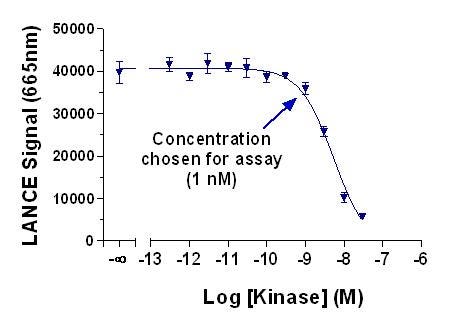
Figure 3. Titration of JAK2 kinase using 50 nM pre-phosphorylated ULight-CDK1 substrate and Eu-anti-phosphotyrosine antibody. No ATP was added to the reaction. This assay was run to determine the optimized concentration of JAK2 kinase to avoid antibody depletion (generic anti-phosphotyrosine antibody binding to phosphorylated tyrosines on activated Jak2).
Troubleshooting
| Problem | Possible cause | Solution |
|---|---|---|
| Low signal | Protocol | Prepare s solution/detection mix just before using. |
| Decrease concentration of EDTA. | ||
| Reaction conditions | Essential enzyme cofactor missing. | |
| Low quality water. Contaminating heavy metal cations at high concentrations interact with the Europium chelate and quench fluorescence. Only use ultrapure laboratory grade water for reagent preparation. | ||
| We do not recommend phosphate-based buffers. | ||
| High background | Too high concentration of ULight™ | Use the recommended optimized concentration (50 nM final concentration in a 10 µL enzymatic reaction). Concentrations above 100 nM will increase the background signal. |
| Instrument settings | Check your instrument settings (refer to Instrument settings on LANCE main technology page). | |
| No specific signal | Reaction conditions | Check that you are using the recommended LANCE Ultra ULight substrate for your kinase. |
| Ensure essential cofactors are included in your kinase buffer. | ||
| Peptide substrate | Many kinases from the MAP kinase pathway do not phosphorylate peptides efficiently. You may need to use a cascade assay or protein substrates. |
Other LANCE kinase formats
There are two alternate LANCE kinase assay formats (Figure 4). In a LANCE classic format, a biotinylated peptide substrate is used. The phosphorylated site is again recognized by an appropriate Europium-labeled anti-phospho antibody. A SureLight™ streptavidin-APC is added to bind the biotinylated substrate, to provide an APC acceptor fluorophore. This is a 3-component kinase assay (Streptavidin-APC, biotinylated substrate, and Eu-anti-phospho antibody), and will require additional assay development steps. This format allows you to use your own biotinylated substrate. You could also use a tagged substrate (GST, His) with one of our anti-tag antibodies. LANCE Ultra kinase assays can also be performed as a 3-component assay, if desired.
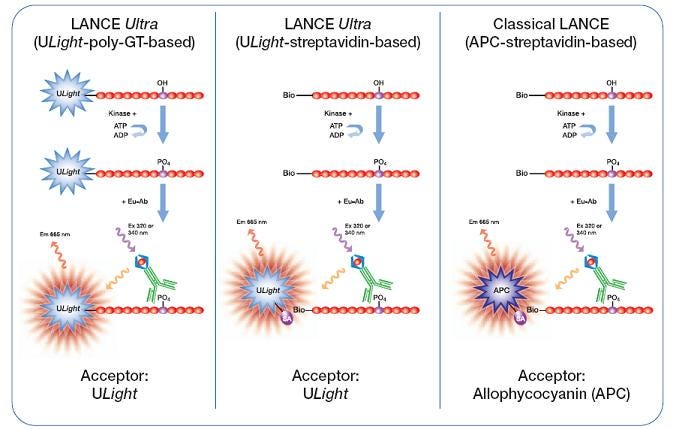
Figure 4. Various LANCE kinase assay formats.
The other alternate format is a LANCE indirect format. In an indirect format, you can utilize your own unlabeled anti-phospho-specific antibody with a LANCE Europium-labeled secondary antibody to provide a donor fluorophore, and your own tagged substrate that can be recognized by an appropriate acceptor-fluorophore labeled catalog reagent.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.
Custom labeling and custom assay development services at Revvity
Revvity offers custom labeling services as well as custom assay development. If you are interested in having your biomolecule custom-labeled, or in custom assay development, please contact our custom teams:
Custom Labeling and Conjugation Services
Custom Assay Development Services





































