

HTRF Human & Mouse Total TBK1 Detection Kit, 500 Assay Points


HTRF Human & Mouse Total TBK1 Detection Kit, 500 Assay Points






The total TBK1 kit detects cellular TBK1 and can be combined with our phospho-TBK1 kit for an optimal readout of the TLR3/4, cGAS-STING pathways
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption and disposal requirements under European REACH regulations (EC 1907/2006).
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
The total TBK1 kit detects cellular TBK1 and can be combined with our phospho-TBK1 kit for an optimal readout of the TLR3/4, cGAS-STING pathways
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption and disposal requirements under European REACH regulations (EC 1907/2006).



HTRF Human & Mouse Total TBK1 Detection Kit, 500 Assay Points



HTRF Human & Mouse Total TBK1 Detection Kit, 500 Assay Points



Product information
Overview
The Total TBK1 cellular assay is designed to monitor the expression level of TBK1 proteins, both phosphorylated and unphosphorylated. It is compatible with our phospho-TBK1 kit and enables the analysis of phosphorylated and total proteins from a single sample for a better readout of T-cell activation. Following pathogen infection, TBK1 can be activated by bacterial LPS and pathogen nucleic acids which trigger the activation of TLR3/4 and cGAS-STING signaling axis.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Oncology & Inflammation
|
| Unit Size |
500 Assay Points
|
Video gallery

HTRF Human & Mouse Total TBK1 Detection Kit, 500 Assay Points

HTRF Human & Mouse Total TBK1 Detection Kit, 500 Assay Points

How it works
Total TBK1 assay principle
The total TBK1 assay quantifies the expression level of TBK1 in a cell lysate. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The total TBK1 assay uses two labeled antibodies: one coupled to a donor fluorophore, the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of TBK1 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the proteins expression under a no-wash assay format.

Total TBK1 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding Total TBK1 HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Total TBK1 1-plate assay protocol
Detection of total TBK1 with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Analysis of total TBK1 to monitor the LPS/TLR4 axis in Kupffer cells
Mouse Kupffer cells (ImKC cell line) were plated in 96-well plates (200,000 cells/well) in complete culture medium and incubated at 37°C - 5% CO2. The day after, cells were treated for 1 hour with increasing concentrations of LPS diluted in serum-free medium. After medium removal, cells were then lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking, and 16 µL of lysate were transferred twice over into a low volume white microplate before adding 4 µL of the HTRF® phospho-TBK1 or total TBK1 detection antibodies. The HTRF signal was recorded after an overnight incubation at RT. LPS induces the activation of TLR4 signaling, leading to a 3-fold increase in TBK1 phosphorylation on Ser172. The expression level of the kinase remains stable which demonstrates that LPS doesn't induce any cytotoxic effect.

Analysis of total TBK1 to monitor the activation of the cGAS-STING pathway
Mouse Kupffer cells (ImKC cell line) were plated in 96-well plates (200,000 cells/well) in complete culture medium and incubated at 37°C - 5% CO2. The day after, cells were treated for 1 hour with increasing concentrations of 23-cGAMP diluted in serum-free medium. After medium removal, cells were then lysed with 50 µL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking, and 16 µL of lysate were transferred twice over into a low volume white microplate before adding 4 µL of the HTRF® phospho-TBK1 or total TBK1 detection antibodies. The HTRF signal was recorded after an overnight incubation at RT. Cell treatment with 23-cGAMP leads to the activation of STING which in turn triggers a 3.6-fold increase in TBK1 phosphorylation on Ser172. As expected, the expression level of the kinase remains constant.

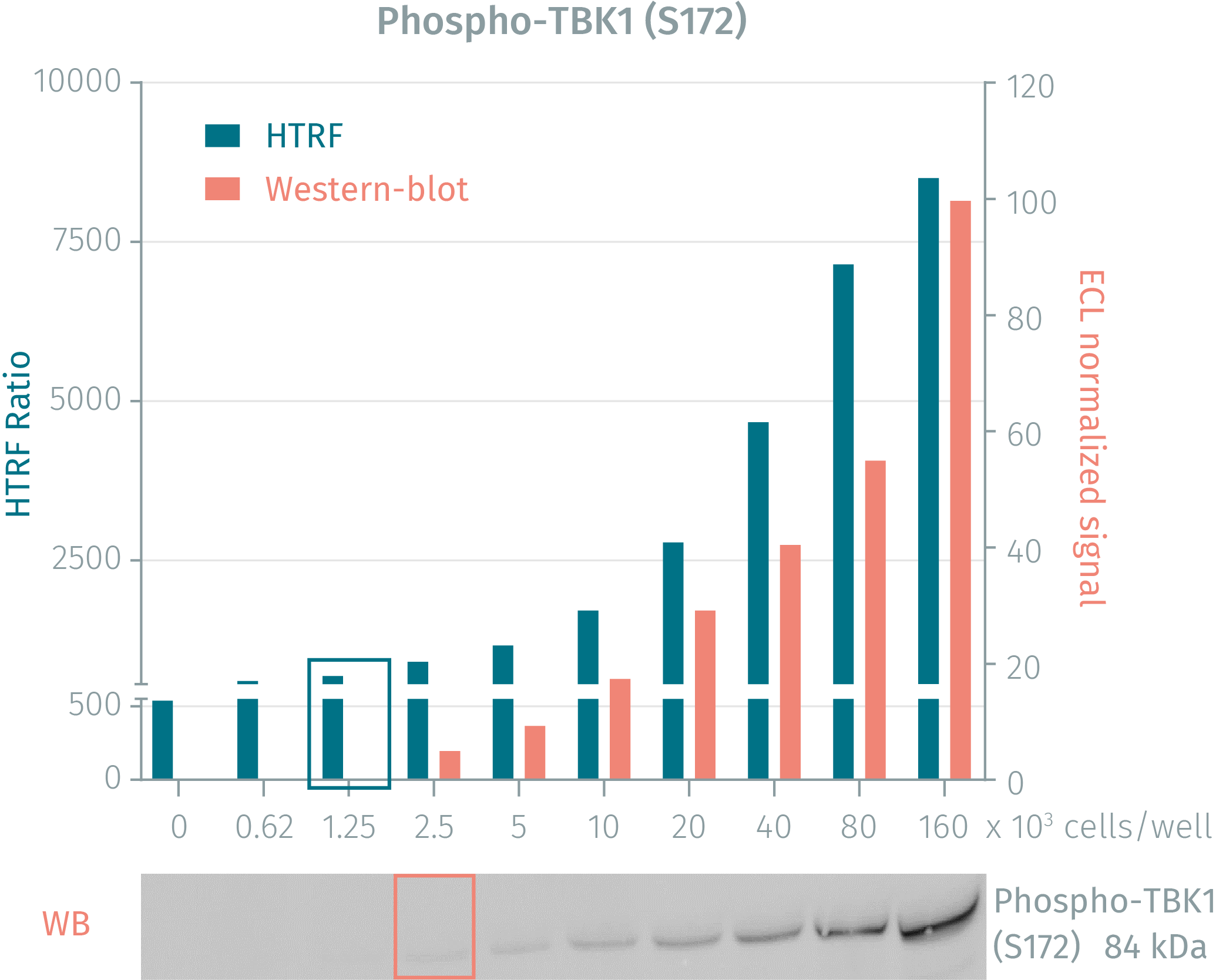
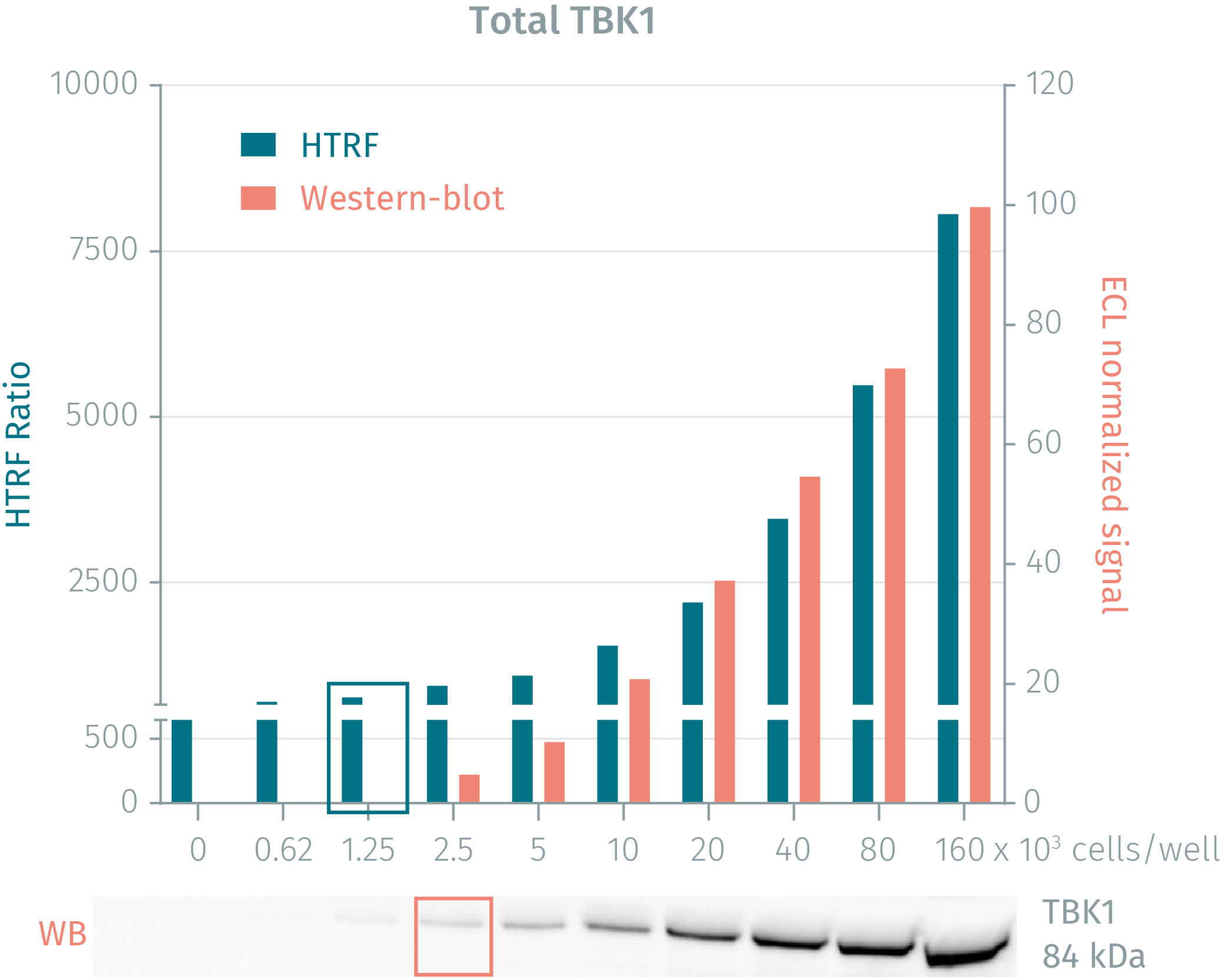
HTRF phospho- & total TBK1 cellular assays compared to Western Blot
The human cervical cancer cell line HeLa was seeded in a T175 flask in complete culture medium, and incubated for 2 days at 37°C, 5% CO2 until 90% confluency was reached. Cells were then treated with 200 nM Calyculin A for 10 minutes and lysed with 3 mL of supplemented lysis buffer #4 for 30 minutes at RT under gentle shaking. Soluble supernatants were collected after a 10-minute centrifugation. Serial dilutions of the cell lysate were performed in the supplemented lysis buffer and 16 µL of each dilution were transferred into a low volume white microplate before the addition of 4 µL of HTRF® phospho- or total TBK1 detection antibodies. Equal amounts of lysates were used for a side by side comparison between HTRF and Western Blot. Using HTRF® Phospho- or Total TBK1 kit, only 1,250 cells/well were sufficient to detect a significant signal while 2,500 cells were needed for minimal ECL signal detection using Western Blot. The HTRF assays are therefore 2 times more sensitive than the Western Blot technique.
Simplified pathway
TLR3/4 and cGAS-STING simplified pathway
TBK1 (TANK-Binding Kinase 1, also known as NAK) is a multimeric kinase activated by autophosphorylation at Ser172. This kinase is a key signaling node between several pathways leading to inflammation and autophagy. Following pathogen infection, TBK1 can be activated by bacterial LPS and pathogen nucleic acids which trigger the activation of TLR3/4 and cGAS-STING signaling axis. Upon LPS binding to TLR4 at the cell surface, the receptor translocates to endosomes and recruits the protein TRAM which in turn activates the protein TRIF. In the same way, dsRNA entering the cell binds to endosomal TLR3 which directly activates TRIF. In the case of dsDNA, it enters the cell and binds to the cytoplasmic sensor cGAS, inducing the production of 23-cGAMP which in turn activates the adaptor protein STING. Further to the activation of each of these pathways, TBK1 is recruited to different signaling complexes and activated by autophosphorylation at Ser172. The kinase in turn activates the transcription factor IRF3 which translocates to the nucleus and induces the expression of type I IFN genes, leading to inflammatory responses. TBK1 is also involved in autophagy where it directly phosphorylates the autophagy receptors optineurin and p62, which target the protein cargo to the autophagosome.

Resources
Are you looking for resources, click on the resource type to explore further.
Get a useful overview of today’s immunity knowledge with this booklet
Immunity is a collection of complex processes involving...
This guide provides you an overview of HTRF applications in several therapeutic areas.
An in-depth review of molecular and cellular pathways
The maintenance of proteostasis, the biological mechanisms that control the...


How can we help you?
We are here to answer your questions.






























