Moving your research from the lab towards the clinic
Immune cell screening services designed by experts to provide the data needed to move research closer to clinical advancements.
Uncover novel targets and make faster therapeutic decisions with reliable in vitro immune cell model data. With our immune cell screening CRO-style services, we offer advanced CRISPR methodologies with functional readouts that allow for the evaluation of physiologically relevant gene edits in primary immune cells. Our ImmuSignature™ assays provide reproducibility across donors for rapid and meaningful strides in compound lead optimization.
Compounds immunogenicity by ImmuSignature screening
Therapeutic lead optimization is a critical and challenging step in drug development that’s often a complex and time-consuming procedure when assessing primary immune cells. Our suite of primary immune cell screening ImmuSignature assays can screen compounds in an in-vitro simulated immune microenvironment, helping to move hits into quality leads from in as little as three weeks.
Collaborate with our preclinical services team at any stage of your project:
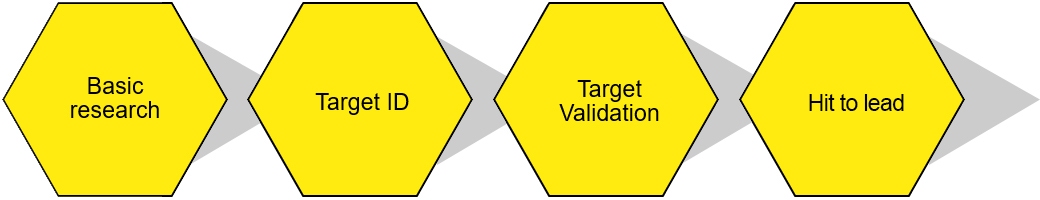
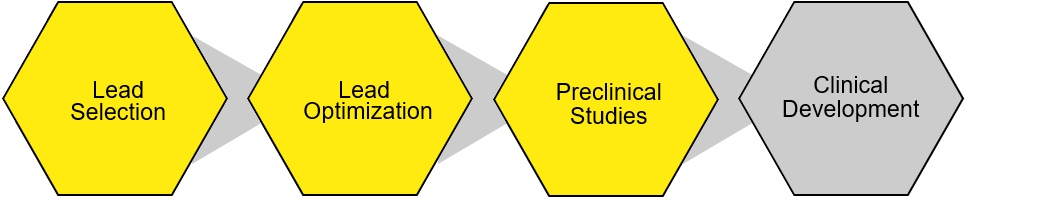
Immune cell screen services projects
Identify relevant genes by genomic CRISPR ko screening in primary human lymphoid and myeloid cells with functional readouts.
- Design guides and identify genes relevant to your model
- Gather data from cell surface markers and protein release
- Explore imaging and functional readouts at high throughput
- Arrayed and pooled modalities in T cells, B cells, macrophages, and monocytes
Compound screening by ImmuSignature assays
Swiftly evaluate the efficacy of lead therapeutics in immune cell-based environments using standardized compound screening assays.
- Analyze the immunological effects of drug candidates
- Assess safety through immunotoxicity analysis
- Obtain dose-response data on T cell proliferation or T reg suppression in weeks
Explore our immune cell screening services
Arrayed myeloid CRISPR ko screens
Pioneer gene modulation on primary immune myeloid cells
Reach the next frontier in microenvironment research with gene editing in monocytes and macrophages.
Pioneer gene modulation on primary immune myeloid cells
Reach the next frontier in microenvironment research with gene editing in monocytes and macrophages.
In early drug discovery, gene editing screening requires highly skilled scientists and substantial resources, particularly when working with primary immune cells. With Revvity’s immune cell screening CRO services, drug developers can unlock new therapeutic potential by leveraging gene editing expertise and preclinical services capabilities in monocytes and macrophages.
Why gene editing on monocytes and macrophages?
Monocytes and macrophages belong to the myeloid lineage and play crucial roles in the immune system, including pathogen recognition, phagocytosis, and the regulation of immune responses. Gene editing in the myeloid lineage can provide insights into disease mechanisms and identify novel therapeutic targets.
However, because of the biological properties of these cell types, cell editing remains a significant challenge when manipulating conditions for synthetic sgRNA transfection.
At Revvity, our preclinical services scientists have successfully optimized Dharmacon™ sgRNA transfection protocols. We are ready to assist drug developers in reaching the frontier of physiological microenvironment research in vitro.
Editing of monocytes or monocyte-derived macrophages (MDMs)
Macrophages, derived from monocytes, play a vital role in the immune system. They can be classified into two main types:
M1 MDMs: Known as classically activated macrophages, they are crucial for pro-inflammatory responses. They help defend against pathogens by producing pro-inflammatory cytokines and effectively targeting microbes and tumor cells.
M2 MDMs: Also known as alternatively activated macrophages, are essential for anti-inflammatory responses and tissue repair. They aid in wound healing, resolve inflammation, and promote tissue remodeling by producing anti-inflammatory cytokines and participating in processes like fibrosis.
Revvity's workflow for editing myeloid cells has been optimized to enhance editing efficiency. Key benefits include:
- Enhanced editing of monocytes/M0 Macrophages.
- Optimized cell culture conditions post-editing, utilizing media containing either M-CSF or GM-CSF.
- Identified the optimal time point for editing efficiency.
- Effective polarization to M1/M2 macrophages.
- Comprehensive endpoint readouts using flow cytometry for M1/M2 polarization cell surface markers and cytokine release by HTRF™.
Arrayed T cell CRISPR ko screens
Unlock the future of therapeutic breakthroughs with T cell arrayed editing
In early drug discovery, gene editing screening requires significant resources, especially with primary immune cells. Leverage T cell arrayed editing for therapeutic development with Revvity's immune cell assay services.
Unlock the future of therapeutic breakthroughs with T cell arrayed editing
In early drug discovery, gene editing screening requires significant resources, especially with primary immune cells. Leverage T cell arrayed editing for therapeutic development with Revvity's immune cell assay services.
CRISPR knock-out gene screening has become commonplace in the dynamic field of drug discovery. It offers the potential for revealing therapeutic targets and understanding genetic disease links. However, a key hurdle is thoroughly understanding the impact of gene suppression on cell function, especially in controlled in-vitro settings. This challenge is amplified when manipulating non-immortalized cells such as primary immune cells with complex gene interplays vital for immune responses.
What is an arrayed CRISPR KO screening in T cells?
It is a specialized genetic screening approach that employs CRISPR-Cas9 gene-editing technology to systematically disrupt or "knock out" specific genes within T cells. In an arrayed screening setup, each sample contains a single KO gene, enabling in-depth analysis of that specific gene with multiplexed readouts, including proliferation (CTG), surface and intracellular markers (FACS), and protein release quantification (HTRF™). The automation and shorter timelines provided by the high-throughput platform allow for the use of cells with a single-gene knockout in downstream functional assays.
CRISPR KO applications in T lymphocytes
- Investigate gene roles in a CRISPR-modulated immune set-up
- Assess compound mode of action in a CRISPR-modulated immune in-vitro model
- Uncover gene-phenotype/cell interactions and study their effects on T cell behavior Advanced miniaturized pipeline for the assessment of drug-phenotype interactions
Revvity`s CRISPR KO T cell screening features
At Revvity, we go beyond traditional approaches by combining the power of CRISPR knockout technology with the reliability of T cell ImmuSignature assays. This distinctive service combination unleashes the potential of our T cell gene screening, offering robust statistical analysis alongside relevant in-vitro immune cell functional assessments to enhance your target discovery efforts.
- Advanced miniaturized pipeline for the assessment of drug-phenotype interactions
- Designed for the screening of bi-specifics, small molecules, or monoclonal antibodies
- Expandable to additional functional read-outs, such as cytokine release through HTRF™ technology
Learn more with the following resources:

Pooled T cell CRISPR ko screens
Undercover novel pathways modulating lymphocytes by analyzing gene loss
Identifying key genes for T cell function is vital for targeting immune disorders. Revvity delivers precise insights to evaluate targets, enhancing the translational impact of your discoveries.
Undercover novel pathways modulating lymphocytes by analyzing gene loss
Identifying key genes for T cell function is vital for targeting immune disorders. Revvity delivers precise insights to evaluate targets, enhancing the translational impact of your discoveries.
Pooled screening, a cost-effective and high-throughput strategy, delivers significant potential in swiftly identifying prospective therapeutic targets. Revvity’s pooled T cell CRISPR KO screens help to effectively tackle challenges in cost, scope, speed, reproducibility, and precision in gene editing. This advancement not only improves our comprehension of T cell biology but also facilitates the discovery of potential therapeutic targets.
What is a pooled CRISPR KO screening in T cells?
It is a powerful method for systematically investigating gene function within a pool of T cells. The process involves designing sgRNA libraries that target a broad collection of genes and transducing primary T cells with a lentiviral vector containing these sgRNAs. The Cas9 enzyme, guided by the sgRNAs, induces double-strand breaks in the target genes, leading to their knockout during cell repair. After expanding the edited T cell population, functional assays are conducted to assess the impact of gene knockouts on T cell behavior. with further multiplexed readouts, including proliferation (CTG), surface and intracellular markers (FACS), protein release quantification (HTRF™), and next-generation sequencing.
CRISPR KO applications in T lymphocytes
- Identify therapeutic targets by systematic gene exploration
- Understand specific gene roles in a CRISPR-based functional screening immune setting
- Assess compound mode of action in a CRISPR-modulated immune in-vitro model
- Uncover gene-phenotype/cell interactions and study their effects on T cell behavior
Revvity`s pooled CRISPR KO T cell screening features
The pooled approach allows simultaneous assessment of numerous genes, accelerating the discovery of novel therapeutic targets with functional relevance in immune-related disorders. This screen enhances reproducibility and reliability by leveraging CRISPR technology, allowing precise gene editing in primary T cells.
- Advanced miniaturized pipeline for the assessment of drug-phenotype interactions
- Designed for the screening of bi-specifics, small molecules, or monoclonal antibodies
- Expandable to downstream assays such as co-culture and functional read-outs, including cytokine release through HTRF™ technology, NGS analytics, and bioinformatics
Learn more with the following resources:
Mixed lymphocyte reaction
Get ready for an IND submission with the ImmuSignature MLR assay
Partner with our specialists for compound screening by Mo-DC: T cell reactivity screening for immunogenicity assessment and make informed decisions for the next step of your project.
Get ready for an IND submission with the ImmuSignature MLR assay
Partner with our specialists for compound screening by Mo-DC: T cell reactivity screening for immunogenicity assessment and make informed decisions for the next step of your project.
What is a Mixed Lymphocyte Reaction assay?
The MLR assay is a platform for testing compounds that modify the interaction between an antigen-presenting cell and T cells to activate, deactivate or repolarize the lymphocyte cell response. This assay allows for testing the efficacy of therapeutics and other immunomodulators. It also provides critical information for immunological responsiveness for drug safety.
The Mixed Lymphocyte Reaction assay (MLR) relies on the principle that T cells from one donor will respond and proliferate in the presence of antigen-presenting cells (APC) from a different donor, caused by a human leukocyte antigen (HLA) mismatch between unrelated donors. The HLA mismatch stimulates the T cells' immune response, inducing them to proliferate. When proteins on the surface of T cells recognize and bind to companion proteins on other cells, immune checkpoints are activated. These checkpoint pathways engage when T cells bind to a professional APC cell, such as a dendritic cell (DC).
Why should I consider the Mixed Lymphocyte Reaction assay for my project?
The MLR constitutes an in-vitro reactivity test that measures the cell microenvironment reaction to a drug helping to predict how the compound would interact with the body. Drug developers use this assay to test the efficacy of therapeutics that potentially increase, decrease, or repolarize the interaction between T cells and the antigen-presenting cells.
Revvity´s standard MLR assay
Our one-way MLR assay enables rapid identification of drugs that regulate T cell activation via allogenic monocyte-derived dendritic cells (Mo-DC). We test your biologics or small molecules at semi-automated high throughput screening in an immune in vitro microenvironment context.
Key advantages:
- Rapid, miniaturized, semi-automated and reproducible 384-well high throughput assay
- Applicable for drug safety, immuno-oncology, and autoimmunity applications
- Providing concise and robust quantitative data in as little as 4 weeks
- Highly enriched human primary T cells and Mo-DC from multiple donors to address donor-to-donor variability with extensive primary immune cell QC
- Multiple physiological readouts with statistically robust data sets, including HTRF, AlphaLISA™ and BioLegend LEGENDplex™ readouts.
Learn more with the following resources:
Macrophages polarization assay
Ignite your myeloid cell research
Research the effects of your therapeutic candidate on the polarization of macrophage subtypes.
Ignite your myeloid cell research
Research the effects of your therapeutic candidate on the polarization of macrophage subtypes.
With a sole focus on supporting your drug discovery research, we’re leading the way in monocyte and macrophage differentiation screening services. Leverage robust M0, M1, and M2 subset generation protocols for reproducible functional assays. Obtain multiplexed data from flow cytometry and cytokine release using our HTRF ™, BioLegend LEGENDplex™, and AlphaLISA™ technologies. Our capabilities in the myeloid lineage allow you to perform compound screening at multiple stages of differentiation.
Discover the functionality of differentiated and polarized macrophages.
Monocyte-derived macrophages (MDMs) type 0 can be efficiently polarized into M1 or M2 MDMs. These specialized cell types are valuable for downstream functional assays, including T cell suppression, and hold potential for groundbreaking advancements in immunology.
Advanced flow cytometry and protein analytics in supernatants are at the heart of our service, providing precise, actionable data. Dive into the intricacies of immune response with confidence using our cell models and analytical tools. With our expert support and resources, you can explore new frontiers in your research.
Expand your immuno-oncology cell research with an in vitro monocyte-derived macrophage pipeline.
Key benefits include:
- Advanced miniaturized pipeline for the assessment of drug-phenotype interactions.
- Screen bi-specifics, small molecules, monoclonal antibodies, and more.
- Expandable to additional functional read-outs, including cytokine release using HTRF technology.
- Seamless integration with CRISPR KO arrayed gene editing projects.
T-cell activation assay
Understand the effect of your therapeutic over T cells with the ImmuSignature TCA assay
Identify the impact compounds have on stimulation and inhibition of helper and cytotoxic T cells to help move into the pre-clinic phase of your drug discovery.
Understand the effect of your therapeutic over T cells with the ImmuSignature TCA assay
Identify the impact compounds have on stimulation and inhibition of helper and cytotoxic T cells to help move into the pre-clinic phase of your drug discovery.
Obtain robust quantitative data from T lymphocytes with a T cell activation assay that removes significant effort for your organization and frees up resources with our T cell activation assay services. As primary T cell activity assays require delicate optimizations, reliable cell supply and solid data analytics, our experts can provide the immunogenicity data you need to help move your project forward with a verified pipeline.
What is a T cell activation assay?
The T cell activation assay is designed to test compounds which can affect the capabilities of T lymphocytes to proliferate. By utilizing co-stimulatory antibodies, the T cell activation assay can mimic the response of CD4+ and CD8+ T cell subpopulation against your compound of interest, including small molecules and biologics.
Why use a T cell activation assay for drug discovery?
Understanding the effect of your developed compounds on T cell activity is critical for immuno-oncology applications. The T cell activation assay is a rapid in-vitro monoculture system to assess the desired effect of your developed molecule to promote or block cell proliferation and activation in CD4+ and CD8+T cell subtypes.
T Cell activation assay service
Our T cell activation assay facilitates quick identification of compounds and novel immunotherapies that can influence the stimulation or inhibition of T lymphocytes in optimized miniaturized screening conditions. We test your biologics or small molecules in a semi-automated high throughput format, helping to move your compounds forward to pre-clinical stages.
Key advantages:
- Short project completion from as little as 3 weeks
- Rapid semi-automated and reproducible 384-well high throughput assay format
- Applicable for immuno-oncology, autoimmunity and drug safety applications
- Robust quantitative data
- Highly enriched human primary T cells from multiple donors to address donor-to-donor variability
- Complementary assay to the Mixed Leukocyte Reaction (MLR)
Learn more with the following resources:
iTreg polarization assay
Explore the effect of therapeutics over inducible T-regulatory cells with ImmuSignature TRP
As working with regulatory T cells is challenging, leverage our scientific expertise in compound screening with immune cells and get data quickly to advance your drug discovery with greater certainty.
Explore the effect of therapeutics over inducible T-regulatory cells with ImmuSignature TRP
As working with regulatory T cells is challenging, leverage our scientific expertise in compound screening with immune cells and get data quickly to advance your drug discovery with greater certainty.
Our iTreg polarization assay supports rapid identification of therapeutic candidates that can control the polarization of naive T cells into a regulatory phenotype. Candidates are run in an optimized miniaturized screening setup that provides consistent and reliable data. Small molecules or biologics are assessed in a semi-automated high throughput configuration, supporting your project with data to help move closer to the clinic.
Key advantages:
- Quick project completion from as little as 4 weeks
- Fast, semi-automated and reproducible 384-well high throughput assay format
- Designed for immuno-oncology and autoimmunity applications
- Highly enriched human primary T cells from multiple donors to address donor-to-donor variability
- Robust quantitative data
What is an iTreg polarization assay?
The iTreg polarization assay helps to determine the effect of therapeutic candidates on the phenotype or functional state of the inducible T lymphocyte regulatory cells. These cells are essential in controlling the immune response and maintaining self-tolerance.
Why use an iTreg polarization assay for drug discovery?
The iTreg polarization assay is a rapid in-vitro monoculture system to assess the desired impact of therapeutic candidates to modulate the iTreg polarization phenotype. For immuno-oncology applications, compounds tested in these assays can demonstrate control of the polarization of a regulatory phenotype. Therapeutics that promote Treg polarization towards an anti-inflammatory phenotype can be tested for their ability to suppress inflammation and prevent disease.
Main Applications of iTreg polarization assay
In drug screening, the iTreg polarization assay can evaluate the potential of a drug to impair iTreg function. This could help treat cancer where inhibiting Treg activity can help boost antitumor immune responses. Also, the assay can test the effectiveness of a drug in enhancing the regulatory phenotype of iTregs, which could be beneficial for treating autoimmune diseases or transplant rejection.
Learn more with the following resources:
iTreg suppression assay
ImmuSignature TRP can evaluate candidates influencing suppression of T effector cells
T cells suppression assay by induced regulatory T cells (iTreg) provides key data insights into the immune microenvironment.
ImmuSignature TRP can evaluate candidates influencing suppression of T effector cells
T cells suppression assay by induced regulatory T cells (iTreg) provides key data insights into the immune microenvironment.
Fast and efficient in vitro functional test to evaluate how compounds affect the function of iTregs (induced regulatory T cells) by enhancing or inhibiting their ability to suppress T effector cells. This assay can be used alongside the iTreg polarization test to better understand compounds' overall impact on the biology of iTregs.
Key advantages:
- Completion of projects from as little as 6 weeks
- Swift and reproducible semi-automated 384-well high throughput assay format
- Specifically designed for applications in immuno-oncology and autoimmunity
- Utilization of highly enriched human primary T cells from multiple donors to account for donor-to-donor variability
- Generation of reliable and quantitative data of high quality
What is an iTreg suppression assay?
ImmuSignature iTreg suppression assay allows for the quick identification of potential therapeutic candidates capable of regulating the suppression of T effector cells. These candidates undergo screening in an optimized miniaturized setup, providing consistent and dependable data. The assay is designed explicitly for evaluating small molecules or biologics. It is performed in a semi-automated high throughput format, bringing you closer to clinical application with substantial data support for the project.
Why use an iTreg suppression assay for drug discovery?
The iTreg suppression assay is an efficient in vitro co-culture method used to evaluate the influence of therapeutic candidates on the modulation of T effector cell suppression through the iTregs. This complex interaction, driven by soluble cytokines, has been shown to regulate the effector component of the adaptive immune system. In the context of immuno-oncology applications, compounds tested in these assays have the potential to disrupt the control exerted by iTregs over effector cells. Conversely, in the case of anti-inflammatory agents, the assay can demonstrate a sustained or enhanced suppression effect.
Primary applications of iTreg suppression assay
In the process of drug screening, the iTreg suppression assay is utilized to assess a drug's ability to hinder the functionality of T effector cells through the interaction with polarized iTreg cells in a co-culture system. This method holds promise for addressing cancer, as suppressing Treg activity could potentially bolster immune responses against tumors. Additionally, this assay enables the evaluation of a drug's capacity to enhance the efficacy of iTregs, which could prove advantageous in the treatment of autoimmune disorders.
Learn more with the following resources:

Custom immune-cell projects capabilities
Overcoming your complex challenges is what we do
Struggling with limited resources or expertise for complex immune cell projects? Partner with our experts to define goals, craft a roadmap, and accelerate the journey to results.
Overcoming your complex challenges is what we do
Struggling with limited resources or expertise for complex immune cell projects? Partner with our experts to define goals, craft a roadmap, and accelerate the journey to results.
When you work with us, you’ll receive frequent and regular status updates, so that you’re informed about the project's progress, milestones, and significant developments, enabling you to make informed decisions and provide timely feedback.
You’ll be provided with a comprehensive and in-depth analysis of results, delivered with our commitment to reliable data. Our rigorous analytical methods deliver accurate and meaningful interpretations, empowering you to make data-driven decisions and drive future strategies.
Benefits of outsourcing with Revvity include
- Increased efficiency: Focus on your competencies and what you do best while we handle specialized tasks.
- Specialized access: Tap into our global talent pool for diverse skills and knowledge.
- Scalability and flexibility: Adjust your operations based on business needs.
- Risk sharing: Transfer certain risks to our capable hands, reducing your burden.
- Time savings: Free up your time for strategic activities while we take care of time-consuming tasks.
Our immune cell custom screening advantages
- Dedicated project management team
- Miniaturized primary immune cell assays platform
- Donor specifications
- Imaging readouts
- Leverage the reliability of ImmuSignature assays
- Extensive library of 600+ cell lines
Examples of assay development areas
Consult with our scientist to develop your next screening assay, including in the following areas of primary immune cells:
- T cell-mediated tumor-killing assays
- B cell polarization and suppression assays
- M1 and M2 macrophages polarization
- NK cell Antibody-Dependent and Cell-mediated Cytotoxicity (ADCC) assays
- Antibody-dependent cellular phagocytosis (ADCP)
- 3D T cell infiltration assay.
- Arrayed B cell screening
- Complement-dependent cytotoxicity (CDC)
Learn more with the following resources:































