Innate immunity
Innate immunity is a collection of non-specific tactics and defenses that can be activated immediately or within a short period of time in response to the presence of antigen. These tactics involve a vast number of receptors (TLRs) and sensors (STING axis and inflammasomes) that trigger inflammatory signals and antigen clearance at the cellular level.
Most nonspecific immune cells, such as dendritic cells, macrophages, neutrophils, natural killer cells, and mastocytes, share these receptors and sensors:
-
Toll-like receptors
TLRs are pattern recognition receptors (PRRs) that bind to ligands that are particular to microorganisms, such as lipids and proteins from bacterial walls or double-stranded RNA. Humans have ten different types, which are expressed on the cell surface (Types 1, 2, 4, 5, 6, and 10) or in intracellular vesicles (3, 7, 8, and 9).
They're employed and researched as active immuno-oncology therapies, as well as infectious and inflammatory illness treatments. Small compounds are used in these therapies to activate or block distinct TLRs and modify the immune response.
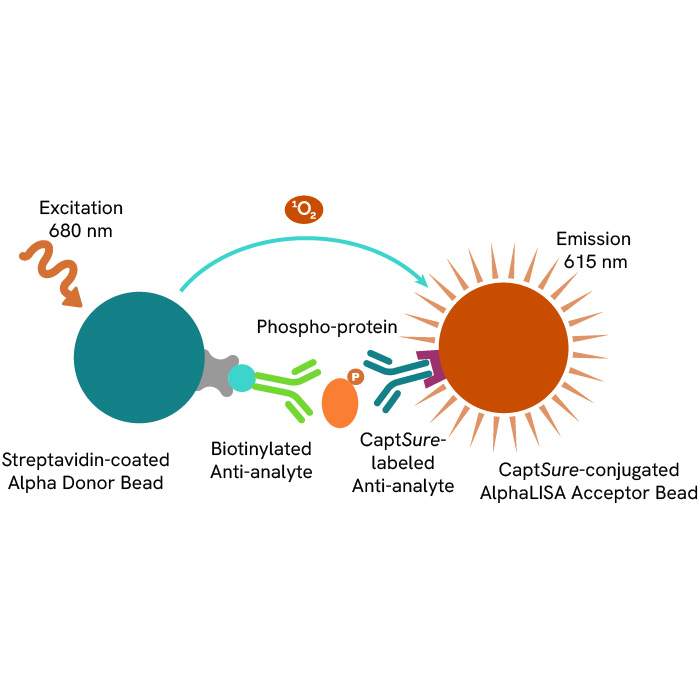
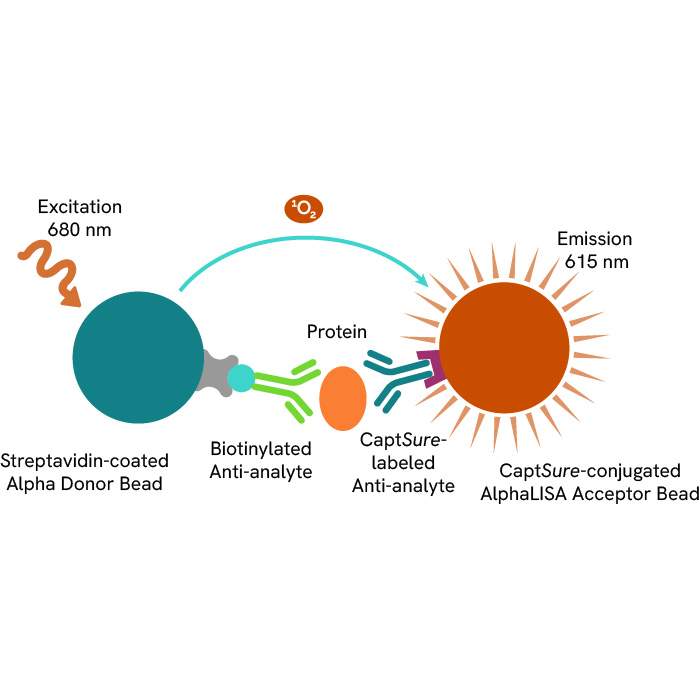
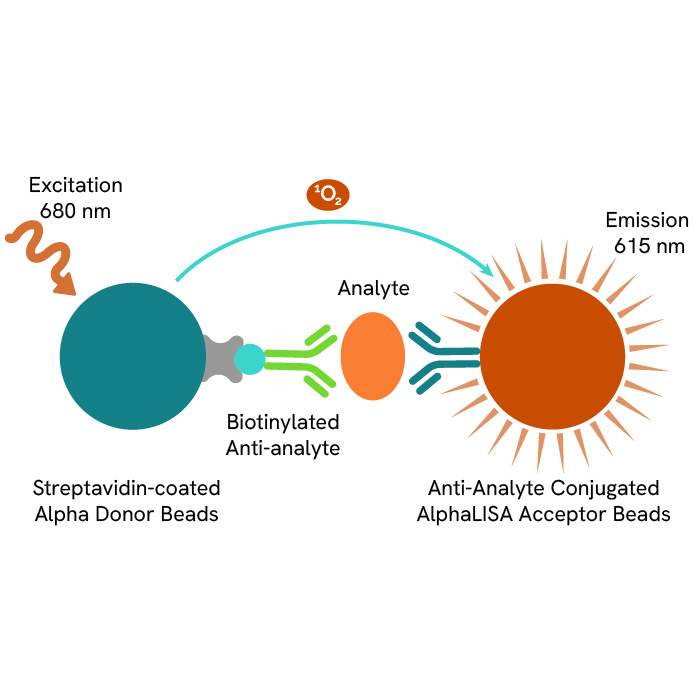
Discover our ready-to-use immuno-oncology reagents for monitoring TCR signaling transcription factors, phosphoproteins, and kinases:
-
STING axis
Stimulator of Interferon Genes (STING) is a cytoplasmic protein that works with the cytoplasmic DNA sensor cGAS to detect floating dsDNA from pathogens or shrinking mitochondria and begin a signal transduction pathway including TBK1, IRF3, and NF-kB to stimulate the release of pro-inflammatory mediators and type 1 interferons (TNF-α, IFN-α, IFN-β).
Tumor-derived DNA fragments have been proven in immuno-oncology to trigger anti-tumor immune responses via the STING pathway and activating it has shown promising anti-tumor benefits in preclinical animals. It's also been studied extensively in the context of autoimmune inflammation, as nucleic acid self-antigens are suspected to cause disorders like lupus and psoriasis.
We’ve developed a panel of high-quality assays to identify those pro-inflammatory signal transduction pathways, as well as the resulting transcription factors and STING activity products:
-
Inflammasomes
Inflammasomes are cytosolic complexes that stimulate the extracellular production of the pro-inflammatory alarm protein HMGB1 and enable the fast activation of cytokines IL-1β and IL-18 from their precursors pro-IL1β and pro-IL18.
They are formed by identical ASC specks that are made up of an upstream sensor protein (NLRP, NLRC, or AIM2) that is coupled to a downstream effector pro-caspase-1 by the adaptor protein ASC. When their sensor proteins bind, this structure allows them to assemble into wheel-like complexes.
Explore our immuno-oncology research tools that target distinct stages of inflammasome activity and end results in order to monitor and better understand these complexes.
Natural killers
Natural killer (NK) cells are a type of lymphoid cell that can attack and lyse a wide range of tumorous, diseased, or injured cells in a non-specific manner without being activated. They also serve as sentinels, alerting adaptive immunity with inflammatory cytokines and detecting degraded cells early on. Due to these qualities, they are crucial early responders to tumor formation.
They are known to target and destroy cells using a group of activating and inhibitory receptors that direct their decision-making process by binding substances expressed on the surface of tumorous or infected cells and MHC I or inhibitory ligands, respectively. They are a prospective research axis that requires a deeper knowledge of their receptor and signaling cascades because of their non-specific lytic characteristics.
Immune checkpoints
Immune checkpoint blockage is a promising way to trigger therapeutic anti-tumor immunity. Tumors co-opt some immune-checkpoint pathways as a significant mechanism of immunological resistance, especially against T cells that are specific for tumor antigens. Because many immunological checkpoints are triggered by ligand–receptor interactions, antibodies can easily inhibit them, or recombinant variants of ligands or receptors can regulate them.
Immunosuppression
Immunosuppression is a term that describes the mechanisms that might reduce or stop the immune response. It's notably crucial in preventing inflammatory signals and killer cell activities from becoming hazardous to the organism after an antigenic threat has been resolved. Regulatory T-cells are usually in charge of immunosuppressive techniques, but other cell types, such as type 2 macrophages, can also inhibit the immune system by secreting TGF-β.
Finding strategies to monitor and reduce immunosuppression in immuno-oncology research could lead to medicines that increase the immune response and empower the immune system to fight tumors by eliminating or altering the brakes that keep it in check.
Our portfolio was created to map the primary immunosuppressive techniques employed by regulatory T-cells, such as the release of anti-inflammatory IL-10, the production of immunosuppressive adenosine, immunological checkpoint-driven inhibition, and TGF-β expression.
Other immune pathways
Other inflammatory pathways are relevant to immuno-oncology research because they provide insight into cell-to-cell signaling, inflammation, and proliferation, in addition to the usual major cell types and signaling pathways that characterize immuno-oncology. Revvity focused on a few of these pathways in order to create more comprehensive and polyvalent solutions. Among them are the P13K/AKT/mTOR axis and the MAPK pathway, which are both involved in cell survival and proliferation and are frequently deregulated in cancer, as well as the NF-kB and JAK/STAT axis, which are involved in cellular inflammation.
Cytokines for cancer research
Cytokines are a collective group of small signaling proteins that offer a window into the regulation of immune and inflammatory responses of the body, providing vital information for furthering oncology research. Cytokines can be classified as pro-inflammatory or anti-inflammatory. Pro-inflammatory cytokines (e.g. IL-1, IL-2, IL-12, IL-17, IL-18, IFN-γ, and TNF-α) play a critical role in regulating the immune system and coordinating cell-mediated immune responses.
Pro-inflammatory cytokines govern immune cell proliferation, activation, differentiation, and homing to infection sites with the goal of controlling and eradicating intracellular pathogens such as viruses. Anti-inflammatory cytokines (e.g. IL-4, IL-10, IL-11, and IL-13) control the pro-inflammatory cytokine response and can counter-regulate production and function of pro-inflammatory cytokines at multiple levels.
Involved in both innate and adaptive immune pathways, and secreted by both host and malignant cells within the tumor microenvironment, cytokines play a complex role in the progression of disease and in the development and efficacy of immunotherapies. Several cytokines are known to limit tumor cell growth by a direct anti-proliferative or pro-apoptotic activity, or indirectly by stimulating the cytotoxic activity of immune cells against tumor cells. Pro-inflammatory cytokines can contribute to cancer immunotherapy, acting on every phase of the cancer immunity cycle. Utilizing cytokines for the development of monotherapies and immunotherapies is a key strategy in the immuno-oncology space.
Explore our comprehensive portfolio of homogeneous cytokine assays for the detection and quantification of interleukins, chemokines, interferons, lymphokines and growth factors.
Suggested content:
- Cytokine research reagents
Featured resources


Filters
1 - 25 of 447 Products and Services
The AlphaLISA™ SureFire® Ultra™ p-ERK 1/2 (Thr202/Tyr204) assay is a sandwich immunoassay for quantitative detection of phospho-ERK 1/2 (phosphorylated on Thr202/Tyr204 in ERK1, or Thr185/Tyr187 in ERK2) in cellular lysates using Alpha Technology.
The AlphaLISA™ SureFire® Ultra™ Total ERK 1/2 assay is a sandwich immunoassay for quantitative detection of ERK 1/2 (both phosphorylated and non-phosphorylated) in cellular lysates using Alpha Technology. This assay is intended to be used as a normalization for phosphorylation studies.
Phospho-IKK-alpha (Ser176/180) assay enables the cell-based detection of Ser176/180 phosphorylation of activated IKK-alpha directly in whole cells.
The HTRF Human Phospho-MET Tyr1234/1235 detection kit is designed to monitor the expression level of cellular MET phosphorylated at Tyrosine 1234/1235.
This HTRF kit detects cellular STAT6 and can be used as a normalization assay with our phospho-STAT6 kit for an optimal readout of JAK/STAT signaling.
The Total IKK-alpha kit monitors the cellular IKK-alpha expression level and can be used as a normalization assay for the phospho-IKK-alpha kit.
Our Membrane Target System: Adenosine A3 (human) membranes are prepared from cells that express recombinant or endogenous receptors. Selected for optimal performance in binding assays.
The AlphaLISA™ Human CXCL1/GROα Detection Kit is designed for detection and quantitation of human CXCL1/GROα in cell culture media or serum using a homogeneous (no-wash steps, no separation steps) assay.
The AlphaLISA™ Human IL-15 Biotin-Free Detection Kit is designed for the quantitative detection of human Interleukin 15 in serum, cell culture medium, and other samples types using a homogeneous (no wash steps, no separation steps) assay. The biotin-free kit is compatible with high-biotin culture media and other sample types that contain high levels biotin.
The AlphaLISA™ SureFire® Ultra™ total MKK4 assay is a sandwich immunoassay for quantitative detection of MKK4 (phosphorylated and non-phosphorylated) in cellular lysates using Alpha Technology. This assay is intended to be a normalization or control assay in conjunction with the AlphaLISA SureFire phospho-MKK4 assay.
The AlphaLISA™ SureFire® Ultra™ Human Phospho-ULK1 (Ser556) assay is a sandwich immunoassay for quantitative detection of phospho-ULK1 (Ser556) in cellular lysates using Alpha Technology.
The AlphaLISA™ Human Interleukin 13 Detection Kit is designed for detection and quantitation of human IL-13 in serum, buffered solution or cell culture medium in a homogeneous (no-wash steps, no separation steps) assay.
This HTRF kit is designed for robust cell-based quantification of AKT2 modulation, phosphorylated on Ser473.
This HTRF kit enables the cell-based quantitative detection of phosphorylated c-RAF Ser43.
The Phospho-BAD (Ser112) kit enables the cell-based quantification of phosphorylated BAD (Ser112) as a marker of cells entering apoptosis.
This HTRF kit enables the cell-based quantitative detection of phosphorylated RSK1 Serine 380.
This HTRF kit enables the cell-based quantitative detection of phosphorylated STAT6 as a result of cytokine induced receptor activation, such as IL4, IL13 or IFNa.
The AlphaLISA™ SureFire® Ultra™ p-MEK1 (Ser218/222) HV (high volume) assay is a sandwich immunoassay for quantitative detection of phospho-MEK1 (phosphorylated on Ser218/222) in cellular lysates using Alpha no-wash technology.
The AlphaLISA™ SureFire® Ultra™ p-SMAD3 (Ser423/Ser425) assay is a sandwich immunoassay for quantitative detection of phospho-SMAD3 (phosphorylated on Ser423/Ser425) in cellular lysates using Alpha Technology.
The AlphaLISA™ SureFire® Ultra™ p-AMPKα 1/2 (Thr172) assay is a sandwich immunoassay for quantitative detection of phospho-AMPKα (phosphorylated on Thr172) in cellular lysates using Alpha Technology.
The AlphaLISA™ SureFire® Ultra™ total JNK1/2 assay is a sandwich immunoassay for quantitative detection of JNK1/2 (phosphorylated and non-phosphorylated) in cellular lysates using Alpha Technology. This assay is intended to be a normalization phosphorylation studies.
The AlphaLISA™ Human C-X-C Motif Chemokine 9 / Monokine Induced by Gamma Interferon (CXCL9 / MIG) Detection Kit is designed for detection and quantitation of human CXCL9/MIG in serum, buffered solution or cell culture medium in a homogeneous (no-wash steps, no separation steps) assay.
The AlphaLISA™ Human C-C Motif Chemokine 3 / Macrophage Inflammatory Protein-1alpha (CCL3 / MIP-1α) Detection Kit is designed for detection and quantitation of human CCL3/MIP-1α in serum, buffered solution or cell culture medium in a homogeneous (no-wash steps, no separation steps) assay.
The phospho-SLP-76 (Ser376) kit enables the cell-based quantitative detection of phosphorylated SLP-76 at Ser376, for monitoring T-lymphocyte activation.
The phospho-ZAP-70 (Tyr319) assay enables the cell-based quantitative detection of ZAP-70 phosphorylated on Ser Tyr319 as a readout of T-cell activation.