Recombinant viral vectors have been modified and re-designed to leverage the natural ability of viruses to introduce genetic material into host cells, while minimizing the risks associated with the wild type disease-causing virus.
Three popular vehicles include Adeno-associated viruses (AAV), Lentiviruses (LV), and Adenoviruses (AV). Each vector type has unique biological characteristics that make it suitable for specific research applications. In this blog, we will discuss considerations for selecting the most appropriate vector for your research needs.
Adeno-associated viruses (AAVs)
What are adeno-associated viral vectors?
AAV is the leading platform for in vivo gene delivery for treatment of various human genetic disorders.
It was first discovered in 1965 as a contaminant of AV preparation and is one of the smallest viruses, belonging to Parvoviridae family. AAV is a non-enveloped virus containing a single-stranded 4.7 kb DNA genome which is packed into an icosahedral capsid of around 25 nm.
AAVs belong to the genus Dependoparvovirus as they replicate only in the presence of helper viruses such as adenovirus or herpes virus. However, widely used techniques are now available for the manufacturing of viral particles without the need for separation from the helper virus.
AAV-based viral vectors are commonly used tools in research and clinical applications because of their relatively low immunogenicity and lower risk for insertional mutagenesis, with the latter being due to their ability to mediate long-term expression from episomal, non-integrated vector expression cassettes. Another feature of AAV-based vectors is that they do carry viral genes and cannot replicate in the target cells.1
AAVs in drug development and preclinical studies
AAV vectors have excelled at in vivo disease modelling due to their long-term gene expression. Persistence of the AAV in its episomal state depends on the cell turnover in the targeted tissue and the number of AAV genomes in the cell. Persistence of AAV episomes over 1.5 years have been reported in tissues with dividing cells, and potentially indefinitely in post-mitotic tissues.2
Within the drug developmental phase, AAV vectors are used for pharmacological profiling and mechanism of action (PoC) studies. Pre-clinical studies may use AAV for:
- Animal pharmacology (biodistribution)
- Dosing and administration route determination
- Safety profiling (IND-enabling toxicology studies)
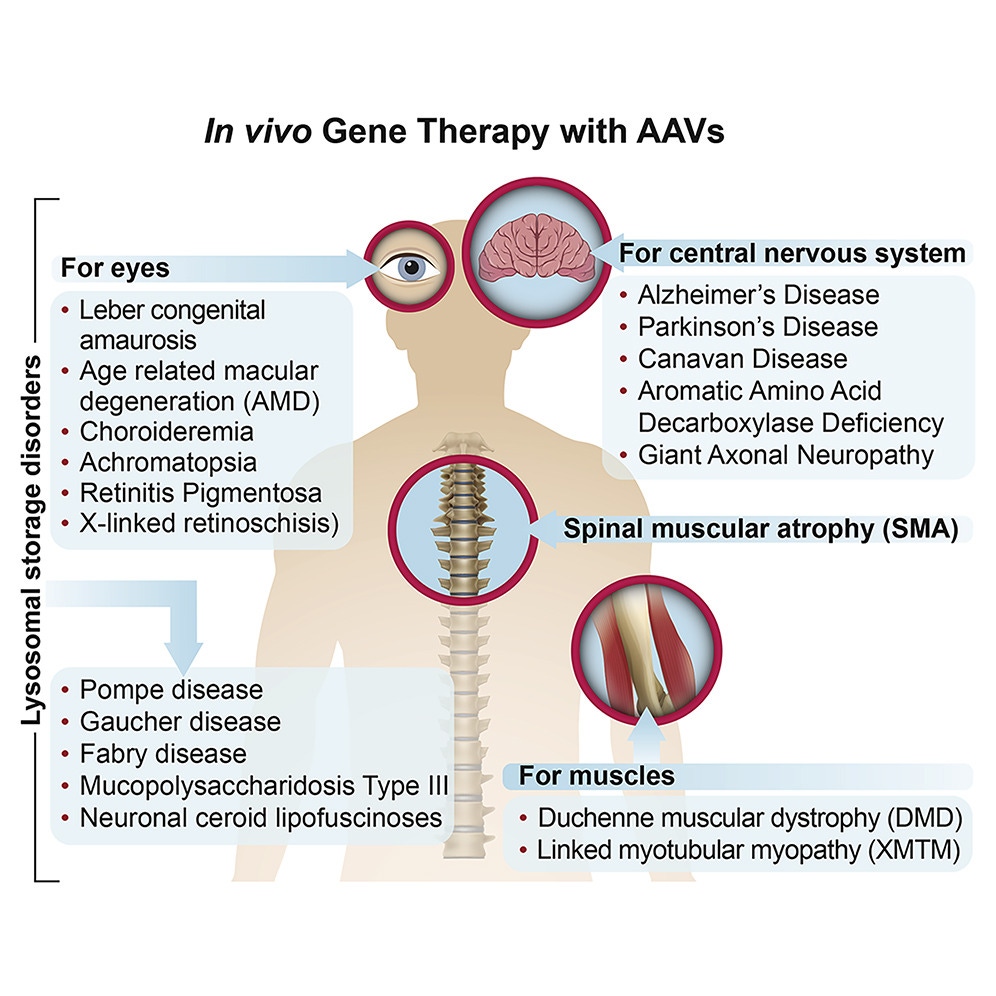
Application of AAVs for in vivo gene therapy
The success story of AAV-based vectors has already led to the clinical approval of patient life-changing drugs, such as Zolgensma® treating the fatal genetic disease spinal muscular atrophy. At the time of writing, there are more than 300 clinical trials3 based on AAV vectors addressing indications such as:
- Muscle diseases such as Duchenne Muscular Dystrophy, Limb Girdle muscular dystrophy, X-linked Myotubular Myopathy, Spinal muscular atrophy
- Neurologic disorders such as Parkinson’s disease, Alzheimer's disease, Dementia, Huntington’s disease, Canavan disease, Aromatic amino acid decarboxylase deficiency, Giant axonal neuropathy
- Metabolic diseases such as Batten disease, Sanfilippo syndrome B, Crigler-Najjar Syndrome
- Ophthalmic diseases such as Leber’s Hereditary Optic Neuropathies, Leber’s congenital amaurosis, Age-Related Maculophathies, Achromatopsia, Retinitis pigmentosa, X-linked retinoschisis

A list of current available gene therapy treatments utilizing AAVs from Mendell et al. (2021)4.
Current AAV trends
The characterization and alleviation of AAV vector interaction with the immune system continue to be a major priority of the field, including vector design for reduced immunogenicity, and clinical management of anti-capsid and -transgene immunity. To allow for more targeted payload delivery with reduced vector dose, various AAV capsid engineering methods have been established, including computational, directed evolution and chemical modification methods.
Due to the risk of insertion mutagenesis associated with LV vectors and increased efforts in improving the safety of gene therapy, AAV is being investigated as an alternative tool for ex vivo modification of T cells for autologous cell transplantation.5
In 2016, transduction of primary human CD8+ T cells and targeted gene knock-in by homology-directed repair genome editing was achieved using AAV serotype 6 for donor delivery.6 Since then, an in vivo chimeric antigen receptor (CAR) T-cell strategy was tested by intravenous dosing the AAV-DJ variant.7
These novel approaches using AAV-mediated in vivo gene delivery to immune cells could change the immunotherapy paradigm.
Technologies and services for AAVs
We offer technologies to support the development of novel capsids as well as preclinical AAV manufacturing services from our expert team.
Learn more about Revvity’s AAV technologies and AAV services.
Lentiviruses (LVs)
What are lentiviral vectors?
Lentiviruses are a subtype of retroviruses which have a single-stranded RNA genome that encode for three major structural genes: gag, pol, and env.
The intensive research on HIV in pursuit of a treatment for AIDS led to the development of recombinant HIV-based vectors. Lentiviral vectors contain only the viral cis-regulatory elements necessary for genome integration, transcription, and packaging of the vector RNA. All viral protein coding regions are removed providing space for insertion of a foreign DNA sequence. Separating the lentiviral genome onto several plasmids reduced the risk of generating replication competent lentiviruses.
In contrast with classical retroviruses, lentiviral vectors have the ability to transduce both dividing and non-dividing cells which are used as general tools for basic research and translational applications.
A milestone was reached in 2017 when the first CAR-T LV-based therapy was approved by the FDA for treatment of refractory or relapse (R/R) B cell precursor acute lymphoblastic leukemia.8
At the time of writing, there are more than 360 clinical trials ongoing worldwide using LV as a genetic delivery tool addressing a range of diseases.9
Application of LVs in cell model development
LV are widely used for generation of cell lines that stably over-express genes of interest or hRNA/shmiR for knock-down approaches.
The resulting modified mammalian cell lines are most commonly used for:
- Functional gene analysis
- Drug screening assays
- Antibody and protein expression
- Study of cellular events such as cell differentiation
- Assay development
- Toxicity studies
Lentiviral vectors in ex vivo gene therapy (cell therapy)
The ability of LV to stably integrate into host genome for long-term gene overexpression made it a promising tool for treatment of monogenetic disease, hematological malignancies and solid tumors.
LV’s most common usage is in adoptive cell transfer therapies where it allows developers to deliver a therapeutic transgene (e.g. CAR or T cell receptors) into the desired cells. One way to classify ex vivo cell therapy is based on the cell type to be modified:
- T cells (CAR or TCR)
- Stem cell
- TILs
- NK & NKT
- Tregs
- Macrophages
- Dendritic cells
Lentiviral application to in vivo gene therapy
Pseudotyping LV with the VSV-G envelope proteins confers it with a wider tissue tropism and more efficient transduction capacity for a variety of cell types. Through direct injection or systemic delivery, LV can be used for gene expression in different organs:
- Liver (in different species: human, NHPs, or dogs)
- Eye (corneal cells in mice or pigs)
- Lungs (mice)
- Skin tissue (major cell types, including fibroblasts, keratinocytes or endothelial cells)
- Brain (oligodendrocytes, Schwann cells, neurons)
Technologies and services for LVs
At Revvity, we offer a range of lentivirus technologies, including lentiviral vector backbones, therapeutic expression cassette development, and surface modification technology, as well as preclinical LV manufacturing services.
Learn more about our services for lentiviral technologies and lentiviral services.
We also offers the proprietary LentiBOOST™ transduction enhancer technology, which improves lentiviral transduction efficiency across a wide range of cell types.
Learn more about the LentiBOOST technology.
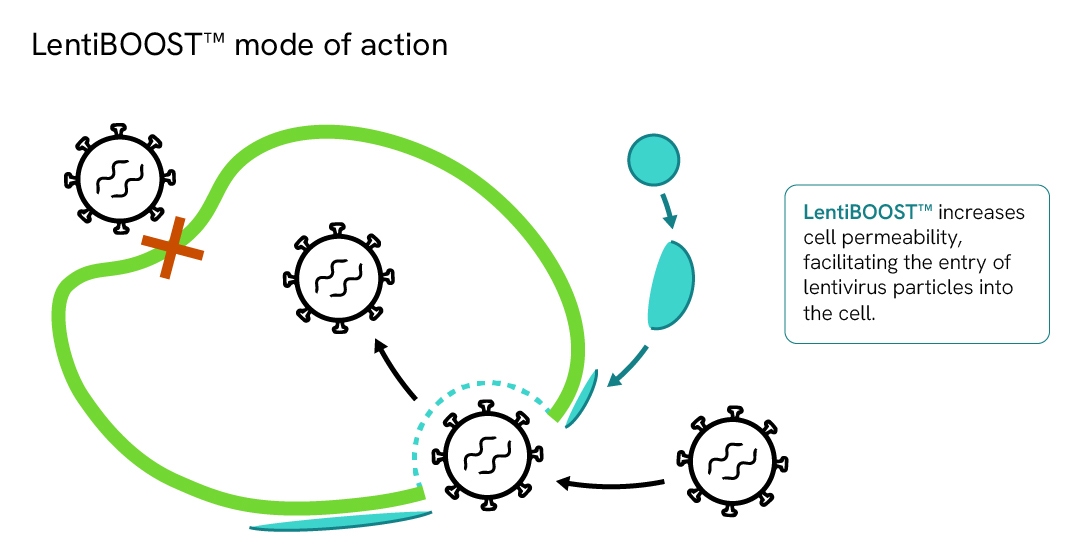
Diagram demonstrating LentiBOOST™ mode of action.
Adenoviruses (AVs)
What are adenoviral vectors?
Adenovirus is a non-enveloped virus with a double-stranded linear DNA genome enclosed in an icosahedral protein shell. It belongs to the family Adenoviridae, and there are 109 species which are sub-classified into six genera.10 Human Adenoviruses are further categorized into seven species (A to G) containing different serotypes. Adenoviruses are ubiquitous and known to cause different conditions, such as infections of the upper respiratory tract, conjunctivitis, gastroenteritis, or pneumonia.11
The adenoviral vectors most commonly used for gene therapies or vaccination are based on the Ad5 serotype, which has a large packaging capacity and is non-toxic and non-integrating. There is a high prevalence of pre-existing anti-Ad5 immunity resulting from natural infection. The attachment and cell entry are mediated via Ad5’s fiber protein interaction with host cell Coxsackievirus and Adenovirus Receptor (CAR – not to be confused with Chimeric Antigen Receptors also abbreviated as CAR above).
To improve safety, our recombinant AV vectors lack three essential genes, E1A, E1B, and E3, found in the wild type virus. Deletion of E1A from the viral vector genome prevents the generation of replication-competent AV vectors so AV vectors cannot replicate in target cells. E1B further helps to inhibit the host cell apoptotic response to adenoviral infection. E1A and E1B are used for viral production and are presented by the virus packaging cells (HEK293). E3 is not essential for virus production.10
Vaccine applications of AVs
AV has the ability to induce strong and sustained innate and adaptive immune responses, particularly T cell-mediated immunity.12 This makes AV an ideal candidate vector for developing vaccines against:
- Infectious diseases (SARS-CoV-2, HIV/SIV, Influenza, Ebola, Tuberculosis, Malaria, MERS, etc.)
- Oncolytic AV3 for cancer treatment (head and neck cancer, breast cancer, and colon cancer)
AVs in research and development
AV vectors exhibit a broad host and tissue tropism with high infectivity, being widely used for transgene expression or knockdown approaches in both in vitro applications and in vivo animal studies.13
Gene therapy applications of AVs
Due to its unique biological features, AVs are used in ongoing gene therapy clinical trials for treatment of diseases such as carotid salivary dysfunction, varicose ulcer, macular degeneration, and myocardial infarction.14
Technologies and services for AVs
Revvity’s cell and gene therapy portfolio includes AV technologies, such as Ad19a/64 vectors, BAC technology, and AdenoBOOST™ transduction enhancer technology.
Learn more about how Revvity’s AV technologies and AV services can support the development of adenovirus vectors for cell and gene therapy applications.
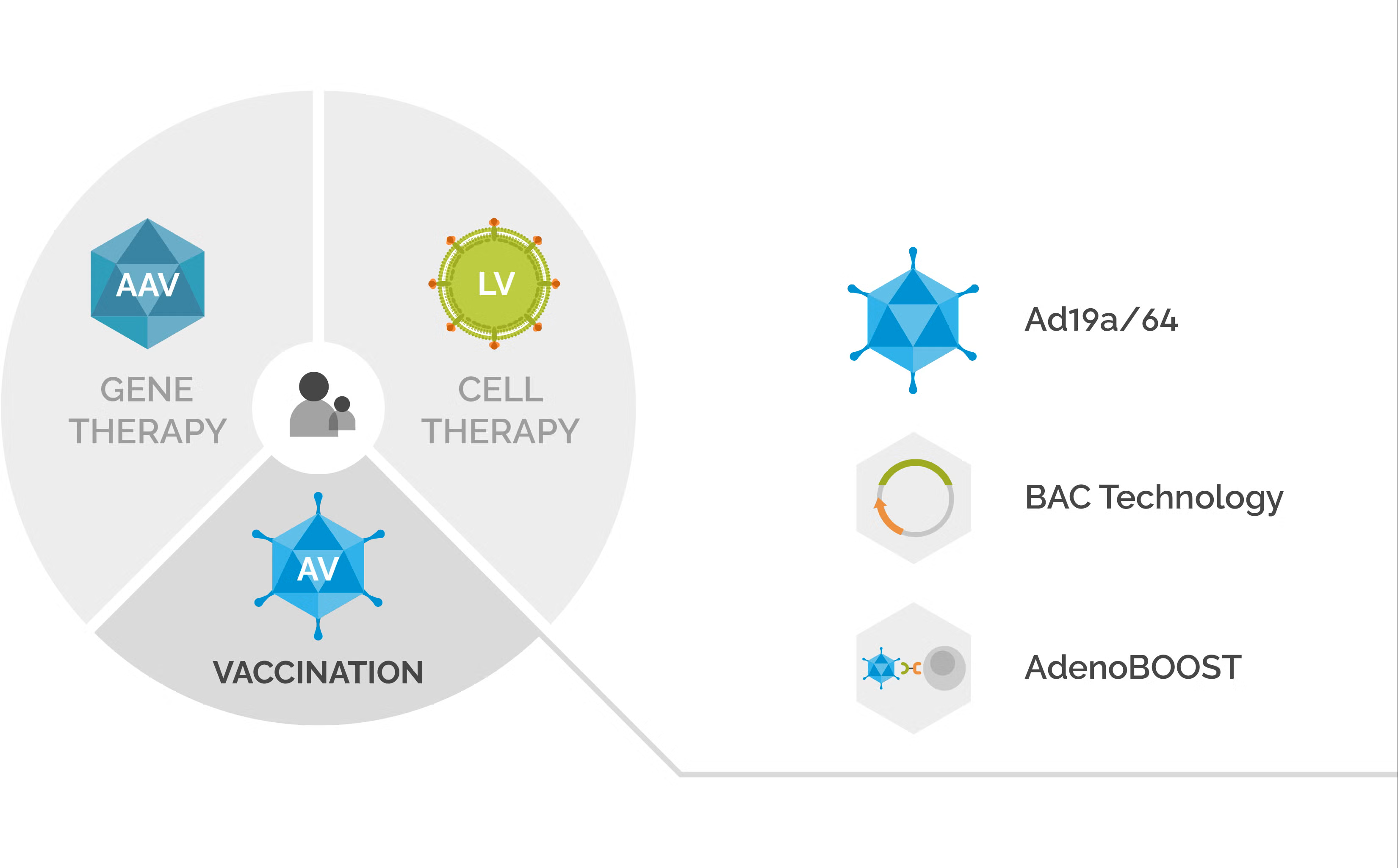
Revvity’s gene delivery portfolio, offers three technology platforms for supporting therapy developers working with adenoviral vectors.
Viral vectors overview
To summarize, here are the key attributes of the viral vectors discussed in this blog post.
| Adeno-associated virus (AAV) |
|
| Lentivirus (LV) |
|
| Adenovirus (AV) |
|
Find out more about how Revvity’s viral vector technologies and services can help advance your research in cell and gene therapies.
References
- Wang, Dan et al. “Adeno-associated virus vector as a platform for gene therapy delivery.” Nature reviews. Drug discovery" vol. 18,5 (2019): 358-378. doi:10.1038/s41573-019-0012-9
- Penaud-Budloo, Magalie et al. “Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle.” Journal of virology vol. 82,16 (2008): 7875-85. doi:10.1128/JVI.00649-08
- Conroy, Gemma. “How gene therapy is emerging from its 'dark age'.” Nature vol. 612,7940 (2022): S24-S26. doi:10.1038/d41586-022-04210-5
- Mendell, Jerry R et al. “Current clinical applications of in vivo gene therapy with AAVs.” Molecular Therapy, vol 29,2 (2021): 464-488. doi.org/10.1016/j.ymthe.2020.12.007
- Milone, Michael C, and Una O'Doherty. “Clinical use of lentiviral vectors.” Leukemia vol. 32,7 (2018): 1529-1541. doi:10.1038/s41375-018-0106-0
- Wang, Jianbin et al. “Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery.” Nucleic acids research vol. 44,3 (2016): e30. doi:10.1093/nar/gkv1121
- Nawaz, Waqas et al. “AAV-mediated in vivo CAR gene therapy for targeting human T-cell leukemia.” Blood cancer journal vol. 11,6 119. 23 Jun. 2021, doi:10.1038/s41408-021-00508-1
- O'Leary, Maura C et al. “FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia.” Clinical cancer research: an official journal of the American Association for Cancer Research vol. 25,4 (2019): 1142-1146. doi:10.1158/1078-0432.CCR-18-2035
- Jadlowsky, Julie K et al. “Long-term stability of clinical-grade lentiviral vectors for cell therapy.” Molecular therapy. Methods & clinical development vol. 32,1 101186. 10 Jan. 2024, doi:10.1016/j.omtm.2024.101186
- “Family: Adenoviridae | ICTV.” Ictv.global, 2024. https://www.ictv.global/report/chapter/adenoviridae/adenoviridae
- Wold, William S M, and Karoly Toth. “Adenovirus vectors for gene therapy, vaccination and cancer gene therapy.” Current gene therapy vol. 13,6 (2013): 421-33. doi:10.2174/1566523213666131125095046
- Zhang, Hongbo et al. “Construction and application of adenoviral vectors.” Molecular therapy. Nucleic acids vol. 34 102027. 9 Sep. 2023, doi:10.1016/j.omtn.2023.09.004
- Wang, Jiang-Hui et al. “Adeno-associated virus as a delivery vector for gene therapy of human diseases.” Signal transduction and targeted therapy vol. 9,1 78. 3 Apr. 2024, doi:10.1038/s41392-024-01780-w
- Jt, Schwartze et al. “Adenoviral vectors for cardiovascular gene therapy applications: a clinical and industry perspective.” Journal of molecular medicine (Berlin, Germany) vol. 100,6 (2022): 875-901. doi:10.1007/s00109-022-02208-0
LentiBOOST GMP Grade: Not for Diagnostic Use. Other applications must be authorized by a license from Revvity.
LentiBOOST Pharma-Grade: For research use only. Not for use in diagnostic procedures.
For research use only. Not for use in diagnostic procedures.