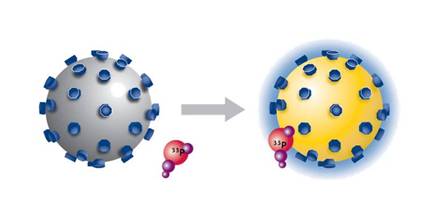
Overview
Scintillation proximity assay (SPA) is a homogeneous and versatile technology for the rapid and sensitive assay of a wide range of biological processes, including applications using enzyme and receptor targets, radioimmunoassays, and molecular interactions. When 3H, 14C, and 125I radioisotopes decay, they release β-particles (or Auger electrons, in the case of 125I). The distance these particles travel through an aqueous solution is dependent on the energy of the particle. If a radioactive molecule is held in close enough proximity to a SPA Scintillation Bead or a SPA Imaging Bead, the decay particles stimulate the scintillant within the bead to emit light, which is then detected in a PMT-based scintillation counter or on a CCD-based imager, respectively. However, if the radioactive molecule does not associate with the SPA bead, the decay particles will not have sufficient energy to reach the bead and no light will be emitted. This discrimination of binding by proximity means that no physical separation of bound and free radiochemical is required.

SPA technology
SPA bead types
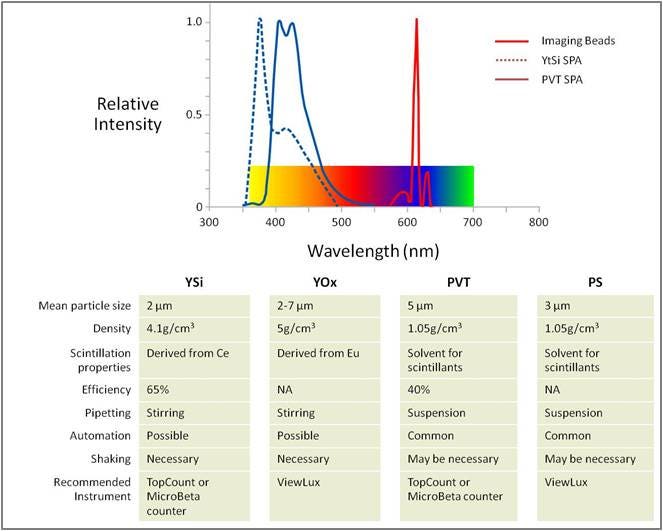
Table 1. SPA bead types
SPA bead types
There are four different SPA beads: two spherical plastic beads (polyvinyl toluene, PVT and polystyrene, PS) and two crystalline beads (yttrium silicate, YSi and yttrium oxide, YOx). The plastic beads are larger in size (5-8 µm) and stay in suspension longer than the crystalline beads (average, 2.5 µm) making them more amenable to automation.
The scintillator in the PVT beads is diphenylanthracine (DPA), which is co-polymerized in the polyvinyl toluene matrix. In YSi SPA beads, naturally occurring cerium ions act as scintillators and are stably trapped within the yttrium silicate crystal lattice. Both scintillators emit a blue light (400 – 450 nm) upon activation. This blue light emission is best captured on a PMT-based scintillation counter (Tri-Carb™ for single tube detection; MicroBeta2™ for microplate detection).
Europium is the scintillator co-polymerized in the polystyrene matrix in the PS imaging beads and stably trapped within the yttrium oxide crystal lattice of the YOx imaging beads. This scintillator emits a red light (615 nm) upon activation. This red-light emission is best captured on a CCD-camera based detector which provides μHTS capability by imaging an entire 384- or 1536-well microplate at one time.
Each bead type can be suitably derivatized for use in several types of SPA applications.
SPA scintillation beads
| Coating | Application | Core bead type | Size | Cat. number |
|---|---|---|---|---|
| Streptavidin | Capture of biotinylated proteins or peptides for use in enzyme or molecular interaction assay | PVT | 50 mg | RPNQ0006 |
| PVT | 150 mg | RPNQ0009 | ||
| PVT | 500 mg | RPNQ0007 | ||
| PVT | 2 g | RPNQ0066 | ||
| PVT | 25 x 2 g | RPNQ0067 | ||
| YSi | 75 mg | RPNQ0015 | ||
| YSi | 250 mg | RPNQ0012 | ||
| Wheat Germ Agglutinin (WGA) | Binds cell membranes and internal components for receptor binding studies | PVT | 100 mg | RPNQ0252 |
| PVT | 500 mg | RPNQ0001 | ||
| PVT | 25 x 500 mg | SPQ0031 | ||
| PVT | 2 g | RPNQ0060 | ||
| YSi | 250 mg | RPNQ0011 | ||
| PVT | 1 g | RPNQ0023 | ||
| WGA PEI Type | Extra coating for reduced non-specific binding | PVT | 500 mg | RPNQ0003 |
| WGA PEI Type | Extra coating for reduced non-specific binding | PVT | 500 mg | RPNQ0004 |
| PVT | 25 x 2 g | RPNQ0065 | ||
| Polyethyleneimine (PEI) | Extra coating for reduced non-specific binding | PVT | 500 mg | RPNQ0097 |
| Poly-L-lysine | Enhances binding of negatively charged membranes | YSi | 1 g | RPNQ0010 |
| PDE | Measure of phosphodiesterase activity | YSi | 500 mg | RPNQ0150 |
| 2 g | RPNQ0024 | |||
| RNA Binding Beads | Uncoated Ysi beads that interact with primary phosphate groups in nucleotides (oligos, DNA and RNA); membranes can also bind | YSi | 500 mg | RPNQ0013 |
| Copper chelate | Capture and assay of His-tag fusion proteins or their binding partners | PVT | 250 mg | RPNQ0095 |
| YSi | 125 mg | RPNQ0096 | ||
| Glutathione | Capture and assay of GST-fusion proteins | PVT | 750 mg | RPNQ0030 |
| YSi | 50 mg | RPNQ0033 | ||
| Protein A | Binding of antibodies via the Fc portion of the antibody for RIA | PVT | 500 mg | RPNQ0019 |
| YSi | 500 mg | RPN143 | ||
| Anti-mouse antibody | Capture of mouse antibody | PVT | 500 mg | RPNQ0017 |
| YSi | 500 mg | RPN141 | ||
| Select-a-Bead kit | Receptor binding studies (contains 100 mg of WGA PVT, WGA Ysi, WGA PEI type A, WGA PEI type B, and Poly-L-lysine beads) | PVT and Ysi | RPNQ0250 |
SPA imaging beads
| Coating | Application | Core bead type | Size | Cat. number |
|---|---|---|---|---|
| Streptavidin | Capture of biotinylated proteins or peptides for use in enzyme assays or molecular interaction assays | PS | 50 mg | RPNQ0263 |
| PS | 500 mg | RPNQ0261 | ||
| YOx | 50 mg | RPNQ0273 | ||
| YOx | 500 mg | RPNQ0271 | ||
| Wheat Germ Agglutinin (WGA) | Binds cell membranes and internal components for receptor binding studies with partially purified cell membrane preps or fractionated, solubilized receptor preps by immobilized receptors attached via glycosylation sites | PS | 50 mg | RPNQ0262 |
| PS | 500 mg | RPNQ0260 | ||
| YOx | 50 mg | RPNQ0272 | ||
| YOx | 500 mg | RPNQ0270 | ||
| WGA PEI Type A | PEI treatment decreases non-specific binding to beads | PS | 50 mg | RPNQ0286 |
| PS | 500 mg | RPNQ0287 | ||
| Polyethyleneimine (PEI) | PEI treatment decreases non-specific binding to beads | PS | 50 mg | RPNQ0297 |
| PS | 500 mg | RPNQ0098 | ||
| Poly-L-lysine | Enhances binding of negatively charged membranes | YOx | 2 g | RPQ0328 |
| Nickel chelate | Capture and assay of His-tag fusion proteins | PS | 500 mg | RPNQ0266 |
| YOx | 500 mg | RPNQ0276 | ||
| Glutathione | Binding and assaying GST-fusion proteins | YOx | 500 mg | RPNQ0278 |
Choosing between SPA Scintillation Beads and SPA Imaging Beads
The choice of SPA bead depends on several parameters, and these are summarized in Table 2.
Table 2. SPA scintillation beads vs. SPA imaging beads
| SPA Scintillation Beads | SPA Imaging Beads | Feature | Benefit | |
|---|---|---|---|---|
| Instrument | PMT readers, standard beta counters | CCD Imagers | SPA Scintillation Beads emit in the blue region and SPA Imaging Beads emit light in the red region of spectra | Match bead to instrument type (e.g. CCD imagers are more sensitive in the red region) |
| Throughput | Medium to high | Medium to ultra high | Choice of throughput | Match instrument and bead choice with throughput needs |
| Format | tubes; 96- or 384-well format | 96-, 384-, 1536-well plates and above | Choice of format | Increase throughput and decrease assay component costs |
| Emission | Blue region (400 nm) | Red region (615 nm) | Choice of emission properties | Reduce color quench caused by orange/red colored compounds through use of SPA Imaging Beads |
Suitable radioisotopes for SPA beads
The most important characteristic when selecting a radioisotope for SPA is the pathlength of the decay particle (Table 3). In general, the shorter the pathlength of the decay particle, the better-suited is it for SPA.
- Tritium and Iodine-125 are ideally suited to SPA
- Carbon-14, Sulfur-35, and Phosphorus-33 have been used successfully with SPA
- Other gamma emitters (Calcium-45, Rubidium-86, Selenium-75, and Cobalt-65 have all been used successfully in SPA assays).
Table 3. Radioisotopes suitable for SPA and their decay particle average pathlengths.
| Isotope | Average pathlength of decay particle in aqueous solution |
|---|---|
| Tritium | 1.5 µm |
| Iodine-125 | 2e-: 1.0 µm, 17 µm |
| Carbon-14 | 50 µm |
| Sulfur-35 | 65 µm |
SPA bead applications
General SPA FAQs
Q. What liquid scintillation counter window settings do I need to use for counting an SPA assay?
A. For counting SPA assays in a liquid scintillation counter or instruments other than the MicroBeta™ counter, set the windows to wide open or count in a ³²P channel. Make certain that you use a blank such as beads plus radioactive tracer, as well as any other assay-specific controls to assess origins of any detectable background.
Q. What is the difference in SPA counting efficiency as compared to liquid scintillation counting?
A. SPA counting is not as efficient as liquid scintillation counting. Typically, counting efficiency of PVT SPA beads will be 40% and YSi SPA beads will be 60% of the expected liquid scintillation counts.
Q. What is the average size of the YSi and PVT SPA beads?
A. The yttrium silicate (YSi) and polyvinyl toluene (PVT) SPA beads range in size from 2-8 µm. The PVT SPA beads average approximately 5 µm in diameter, while the YSi beads are irregular-shaped crystals averaging 2.5 µm in diameter.
Q. How does SPA work with gamma emitters?
A. [125I] and other gamma emitters decay by a process termed "electron capture". This type of decay gives rise to particles named Auger (pronounced 'oh-zhay') electrons and these electrons are detectable by SPA beads. The most common gamma-emitters that have been used in SPA include Iodine-125, Calcium-45, Rubidium-86, Selenium-75, and Cobalt-65.
Q. What is the maximum centrifuge speed I can use to spin down the SPA beads?
A. As a rule of thumb treat the SPA beads as you would cells, spinning at no more than 2,000 rpm in a standard centrifuge without braking. Though higher speed or centrifugal g-forces will not affect the integrity of the SPA bead itself, it is not recommended since the molecular interactions of interest may not be stable to such conditions.
Q: How are proteins and other molecules linked or coated onto SPA beads?
A. Proteins or other macromolecules are covalently linked to SPA beads (PVT, PS, YSi, or YOx) in one of two ways:
- By direct coupling of proteins to the chemically activated surface
- By pre-coating the naked bead with polylysine or polyethyleneimine (PEI), followed by chemical cross-linking of the macromolecule.
Q. What kind of plates do I use with my SPA assays?
A. Most any type of microplate can be used for SPA. However, plates that have high reflective optics and low associated phosphorescence are preferable-these plates are typically opaque white. If you are using a bottom reading instrument, the use of white-walled plates would be best. Additionally, there are plates available that may be used to reduce the non-specific binding of radiolabeled ligand to plate walls which could cause an increase in background signal. They may not be useful for all applications but should be considered as a tool that may reduce the non-specific binding of your labeled compound to the microplate well.
Q. What fluor or scintillator is present in the PVT and YSi SPA beads?
A. The scintillator in the PVT beads is diphenylanthracine (DPA), which is co-polymerized in the polyvinyl toluene matrix. In YSi SPA beads, naturally occurring cerium ions act as scintillators and are stably trapped within the yttrium silicate crystal lattice.
Q. What is meant by the term 'non-proximity effect' and how can it be minimized?
A. A non-proximity effect, or "NPE", is low-level background signal caused by stimulation of SPA beads by unbound radioactivity in solution. NPE generally can occur with higher energy isotopes such as 33P, 35S, and 14C. The potential for NPE can be minimized by maximizing the ratio of solution to beads- mainly by centrifuging or allowing the beads to settle prior to counting, and by increasing the volume of the assay.
Q. What is the mechanism of cell membrane binding to Wheat Germ Agglutinin and poly-lysine SPA beads?
A. Cell membranes couple to the wheat germ agglutinin (WGA) coated SPA beads through interactions between WGA and N-acetylglucosamine residues that are present in glycosylated cell surface proteins. In the case of poly-lysine coated SPA beads, salt-bridges form between the positively charged poly-lysine sites on the bead and the negatively charged lipids that comprise the cellular membrane.
Q. Why are some SPA beads coated with PEI along with WGA?
A. SPA (Scintillation Proximity Assay) beads may be coated with WGA (wheat germ agglutin) alone, or in concert with the agent PEI (polyethyleneimine). The PEI helps minimize non-specific background.
Custom services
Revvity offers custom radiochemical, SPA beads, plate barcoding, and other services. If you are interested, please contact us.
Radiosynthesis and Labeling Custom Services
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























