Overview
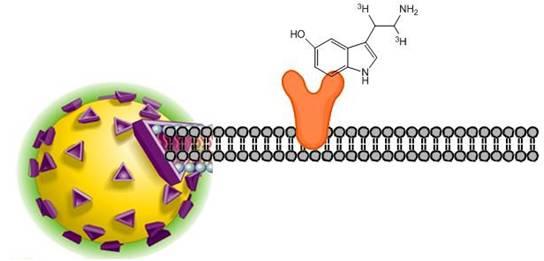
Receptor-binding SPA assays can be configured to determine receptor kinetics, saturation binding, or to detect inhibitors of radioligand binding. SPA has been successfully applied to receptor-binding assays by immobilizing receptors directly to SPA beads via a number of coupling methods. In the SPA format, cell membrane or receptor is captured onto SPA beads. The principle of receptor-binding scintillation proximity assays involves radiolabeled ligands binding to a receptor immobilized on the surface of a SPA bead. The bound ligand is held in close enough proximity to the bead to stimulate scintillant within the bead to emit light. Unbound radioligand is too distant from the bead to transfer energy and therefore goes undetected.

SPA radioligand binding assay
SPA is compatible with receptors prepared from a number of different sources
- Cell membrane preparations from tissue
- Cell membrane preparations from cultured cells
- Whole cells
- Soluble purified receptors
- Solubilized receptors from tissues and cultured cells
When selecting a radioligand, there are a few factors to keep in mind:
- High specific activity: Radioligands with a high specific activity are well-suited for ligand binding assays. Specific activity indicates how much radioactivity there is per molecule of ligand and is usually given in units of Curies per millimole of ligand. Several of our 125 I-labeled ligands are offered at maximum specific activity (2200 Ci/mmol if one 125 I labeling site is available, 4400 Ci/mmol if two 125 I labeling sites are available, etc.). This indicates that virtually every molecule of ligand provided in the stock vial is radiolabeled. For tritiated ligands (3H ligands), you should ideally pick a ligand that has a specific activity above 20 Ci/mmol. The maximum theoretical specific activity per tritium is 29 Ci/mmol (Curies per millimole of tritium). Specific activities above this value indicate that on average, each molecule of ligand has at least one tritium. More guidance can be found in the first section of our Tips and FAQs section below.
- Low non-specific binding: Hydrophobic ligands will generally show higher non-specific binding. Including BSA, salts or detergents in the wash or binding buffer can help reduce non-specific binding. If the stock radiochemical is packaged in a silanized vial, this may indicate the ligand is somewhat hydrophobic.
- High purity: Ideally, the ligand should have a radiochemical purity above 90%. Radiochemical purity decreases over time, and the actual rate of this degradation accelerates over time. Radiochemical purity and degradation rates for our radiochemicals can be found on the lot-specific technical data sheets.
- High selectivity: The more selective the ligand is for your receptor, the better your data will be. High selectivity indicates the ligand will mostly be recognized by only one receptor-of-interest, over other receptors that may be present in a cell membrane. Also, using membranes that over-express the receptor of interest may help reduce any potential contribution from receptors endogenously expressed in the membrane.
Stability: If you need to use your radioligand over an extended period, stability may be a factor for you. 125I-labeled ligands should generally be used within one to two months of the manufacture date. Tritiated ligands should usually be used within 3-6 months of manufacture date; however, there are exceptions to this. Degradation rates and manufacturing dates can be found on our lot-specific technical data sheets. You can also contact technical support to discuss the recommended use time for each Revvity radiochemical.
What do I need to run this assay?
- Solubilized receptors, or cell membrane expressing receptor of interest (Revvity carries receptor-transfected cell membranes)
- Radiolabeled ligand
- Unlabeled ligand as control for non-specific binding
- Ligands and test compounds as appropriate
- SPA beads (See Products and catalog numbers section below)
- Microplates (Refer to table in next section for catalog numbers)
- TopSeal™-A adhesive plate seal
- Appropriate detection instrument (We recommend a MicroBeta™ Microplate Scintillation Counter for SPA scintillation beads, or a CCD Imager for SPA imaging beads.)
Products and catalog numbers
SPA beads
| Bead type | Coating | Core bead type | Size | Catalog number |
|---|---|---|---|---|
| Scintillation | WGA | PVT | 500 mg | RPNQ0001 |
| WGA | PVT | 100 mg | RPNQ0252 | |
| WGA PEI Type A | PVT | 500 mg | RPNQ0003 | |
| WGA PEI Type B | PVT | 500 mg | RPNQ0004 | |
| Polyethyleneimine (PEI) | PVT | 500 mg | RPNQ0097 | |
| Protein A | PVT | 500 mg | RPNQ0019 | |
| WGA | YSI | 250 mg | RPNQ0011 | |
| Polylysine | YSI | 1 g | RPNQ0010 | |
| Protein A | YSI | 500 mg | RPN143 | |
| Select-a-Bead Kit1 | PVT, YSi, PEI type A, PEI type B, and Poly-L-lysine beads | 5 x 100 mg | RPNQ0250 | |
| WGA | PS | 500 mg | RPNQ0260 | |
| WGA | PS | 50 mg | RPNQ0262 | |
| WGA PEI Type A | PS | 50 mg | RPNQ0286 | |
| WGA PEI Type A | PS | 500 mg | RPNQ0287 | |
| Polyethyleneimine (PEI) | PS | 500 mg | RPNQ0098 | |
| SPA Imaging Select-a-Bead Kit2 | PS, YOx, PEI type A, PEI type B, PS and poly-L-lysine beads | RPNQ0291 |
1 Contains 100 mg each of WGA PVT; WGA YSi; WGA PEI Type A; WGA PEI Type B; and poly-L-lysine to allow quick and convenient receptor assay development using SPA scintillation beads
2 Contains 50 mg each of WGA PS; WGA Yox; WGA PEI Type A PS; WGA PEI Type B PS; PEI-coated PS, and poly-L-lysine PS beads to allow quick and convenient receptor assay development using SPA imaging beads
SPA bead types and coatings used for receptor-binding assays
- Receptor-binding SPA assays are usually formatted using wheat germ agglutinin (WGA)-coated SPA beads. WGA binds N-acetyl-s-D-glucosaminyl residues, N-acetyl-s-D-glucosamine oligomers, and glycoproteins present in cell membranes to capture cell membranes expressing the receptor of interest.
- The use of polyethyleneimine to either treat WGA or as a separate bead coating can further reduce non-specific binding
- The treatment of PVT-WGA SPA beads with positively charged polyethyleneimine (PEI) blocks potential non-specific binding sites on the SPA bead surface. There are two SPA bead types available with PEI treatment. The PVT-WGA-PEI type A SPA beads (RPNQ0003) are treated with PEI prior to the coupling of WGA to the PVT SPA bead. The PVT-WGA PEI type B SPA beads (RPNQ0004) are treated with PEI after the WGA coupling stage. The PVT-WGA-PEI type A and type B SPA beads exhibit different characteristics regarding the non-specific binding of radiolabeled ligand directly to the SPA bead. Therefore, both bead types should be evaluated when deciding which SPA bead to use and both are included in the Select-a-Bead Kit. The binding capacity of both bead types for cell membrane protein remains 10–30 μg membrane protein per milligram of SPA bead.
- Poly-L-lysine is used for binding negatively charged cellular membranes.
- Soluble and solubilized receptors can be captured onto antibody binding SPA beads via an antibody specific to the receptor.
- The receptor protein can be biotinylated and captured onto streptavidin-coated beads.

SPA bead types
Plates
| Product name | Well format | Plate color | Number of plates | Catalog number | |
|---|---|---|---|---|---|
| OptiPlate™ | |||||
| 96-well | White | 50 | 6005290 | ||
| 200 | 6005299 | ||||
| 384-well | White | 50 | 6007290 | ||
| 200 | 6007299 | ||||
| 1536-well | White | 50 | 6004290 | ||
| IsoPlate (white walls, clear bottom for bottom read) | 96-well | White walls, clear bottom | 50 | 6005040 | |
| 200 | 6005049 |
Protocols-in-brief
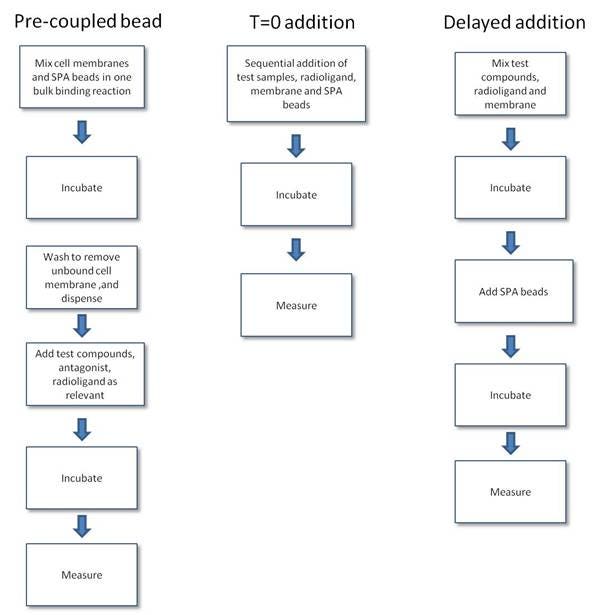
- Pre-coupled format. In this format, the bead and membrane are pre-mixed to form a capture reagent. Excess receptor membrane prep should be removed by washing to maximize binding to coupled receptor vs. free unbound receptor. The pre-coupled bead format reduces the number of additions made to the assay as the beads and membranes are added as a single reagent. One should be aware that orientation of membrane binding is not controllable, and hence not all receptors bound to the bead may be in an orientation capable of binding ligand.
- Simultaneous addition ("T=0") format. In the T=0 format, the ligand, receptor, and bead are added as separate, sequential additions at the start of the assay. The dispensing of the reagents is simple, and the assay format is similar to a filtration format. The T=0 and delayed addition formats are the least complicated formats to use when optimizing the membrane and bead amounts in the assay. In general, a slight excess of bead over membrane is required to ensure complete capture of all the cell membrane present in the assay. The incubation time needed to reach equilibrium may be somewhat longer as compared to the delayed addition format.
- Delayed addition format. This assay format is another format which closely approximates the common filtration format or standard solution binding assay formats used for receptor binding. SPA bead is added to the assay after the ligand has bound to the receptor, thus avoiding the bead interference with ligand binding. The incubation times for this format may also be slightly shorter than the T=0 addition format. Once the receptor ligand assay has equilibrated, the beads are added and a further 30 - 40 minutes are required to ensure that the bead captures the membrane.
SPA radioligand binding protocol-in-brief
Assay optimizations
1. Assay buffer optimization
-
Identify appropriate starting buffer from literature sources or based on experience with similar receptors. Binding assays may require CaCl2, MgCl2, NaCl or other agents added to fully activate the receptor. pH is generally between 7.0 to 7.5. Commonly used buffers include TRIS or HEPES at 25 mM to 100 mM. Protease inhibitors may be required to prevent membrane degradation.
The following are possible factors that can be investigated in a statistically designed experiment to improve radioligand binding to membrane receptor or reduce radioligand binding to SPA beads. The optimization of the assay buffer may be an iterative process in conjunction with the optimization of the assay conditions to achieve acceptable assay performance. Typical concentrations or concentration ranges for some reagents in the tables below. Other reagents may be required depending on the individual receptor/ligand system.
| Agents which reduce NSB | Concentration range |
|---|---|
| BSA | 0.05% - 0.3% |
| Ovalbumin | 0.05% - 0.3% |
| NP-40 | 0.05% - 0.3% |
| Triton X-100 | 0.05% - 0.1% |
| Gelatin | 0.05% - 0.3% |
| Polyethylenimine | 0.01% - 0.1% |
| CHAPS | 0.5% |
| Tween-20 | 0.05% - 0.1% |
| Fetal bovine serum (FBS) | up to 10% |
| Antioxidants/Reducing Agents | concentration |
|---|---|
| Ascorbic acid | 0.1% |
| Pargyline | 10 µM |
| DTT | 1 mM |
| Reduce SPA settling effects | Concentration |
|---|---|
| Glycerol | 10-20% |
| Glucose | 10 mM |
| Polyethylene glycol(PEG) | 5-10% |
| Divalent Cations | Concentration range |
|---|---|
| Magensium (Mg2+) | 1 mM - 10 mM |
| Sodium acetate | 10 mM - 50 mM |
| Calcium (Ca2+) | 1 mM - 10 mM |
| Zinc (Zn2+) | 10 µM - 50 µM |
| Other buffer additives | Concentration range |
|---|---|
| NaCl | 100 mM - 150 mM |
| KCl | 5 mM - 80 mM |
| TRIS | 10 mM - 50 mM |
| HEPES | 5 mM - 100 mM |
| Phosphate buffer | 20 mM |
| pH | 7.0 - 8.0 |
| Aprotinin | 500 units/ml |
| EDTA | 0.5 mM - 5 mM |
2. Selection of assay format
- Pre-coupled bead: coupling the membranes to the beads prior to assay; membrane-to-bead ratio is carefully optimized and hence lower NSBs and better signal-to-noise ratios
- T=0 addition: sequential addition of test samples, radioligand, membrane and bead as separate additions; excess bead is required to capture all the membranes
- Delayed addition: test samples, radioligand and membrane are allowed to equilibrate prior to addition of beads; disadvantage of this approach is that the addition of the SPA bead, after pre-equilibration of ligand and receptor preparation, causes an increase in volume and hence a reduction in the concentrations of the other assay components. This causes a shift in the assay equilibrium
3. Optimization of membrane-to-bead ratio: the key part of the assay development process is the optimization of both the membrane bead ratio and the actual amounts of these components required to achieve the desired signal to noise ratio. The objective is to obtain a signal for further optimization.
- Precoupled bead: incubate varying concentrations of membrane with a fixed amount of bead; aliquots are taken and B0/NSB values are measured
- T=0 addition: set up a matrix varying the quantities of membrane and bead; B0/NSB values are measured
- Delayed addition: set up a matrix varying the quantities of membrane and bead; B0/NSB values are measured
- In these experiments a fixed quantity of radioligand is added; this quantity will depend on the affinity of the radioligand for its receptor and should be a concentration at or around Kd. For 125I ligands, typically quantities of membrane protein between 1 and 100 μg with bead weights between 0.5 and 2.0 mg per well should be investigated. The corresponding figures for 3H ligands are 10 to 100 μg membrane protein and up to 4.0 mg of bead per well. These differences essentially reflect the relative specific activities of radioligands labelled with these isotopes.
4. Optimization of ligand concentration: the volume of the assay buffer and concentration of ligand is varied to optimize the signal and can easily be performed in a matrix format. The objective of varying these parameters is:
- to maximize the signal to noise ratio
- to increase the signal in systems where the affinity of the ligand for the receptor is relatively low.
- This can be achieved by either using increased quantities of labelled ligand and/or decreasing the assay volume, but a consequence is that the non-proximity effect (NPE) component of the NSB may be increased; also, sensitivity may be affected.
5. Determine nonspecific binding (NSB): determine counts bound to bead (label/bead NSB) and NSB in presence of membrane
- Combine bead, buffer and label - Ligand at Kd, 1 mg bead
- Combine label, bead and membrane - Ligand at Kd, 1 mg bead, 10 μg membrane, 10X Kd cold ligand for NSB
6. Examine non proximity effect (NPE): signal generated from non-bound label or ligand
7. Determination of counting window: time course experiments should be performed to establish both the incubation time required for the attainment of equilibrium and the stability of the SPA counts at equilibrium
8. Assay validation
- Perform control experiments using a cell line or tissue which is known not to express the receptor of interest.
- Perform competitive binding curves with known drugs (if available) for the receptor of interest and compare IC50/Ki values.
- Perform saturation binding experiments and compare Kd values.
- Assess assay variability by %CV or Z.’
- To obtain Kd values it is necessary to convert SPA counts to dpms.
Posters, guides, and other resources
- SPA bead technology knowledge base page contains more information on SPA assays, bead types, and instrumentation
- NIH Assay Guidance Website : contains information on assay development
- IUPHAR database - contains receptor-specific information on ligand affinities, lists of ligands that work with various receptors, and other information
Tips and FAQs
- For studies where the receptor density is high, either 3H or 125I-labeled radioligands may be used if the affinity for the receptor is also high (< 10 mM). But, if the affinity of the ligand for the receptor is low (in the range of 10 nM – 10 μM), 125I is probably the radioisotope of choice for labeling.
- If using 3H-labeled ligands with low specific activity (20 – 80 Ci/mmol) then higher expression levels are required (500,000 receptors per cell) corresponding to densities greater than 2 pmoles receptor protein/mg of membrane protein.
- If using 125I-labled ligands with high specific activity (~ 2,000 Ci/mmol) then lower expression levels can be tolerated (50,000 receptors per cell) corresponding to densities of approximately 200 fmol receptor protein/mg of membrane protein.
- Protease inhibitors help prevent the degradation of the receptor or the ligand during the longer equilibration periods often required
- Glycosylated ligands will bind nonspecifically to WGA-PVT beads
- Poly-L-lysine beads associate via an electrostatic interaction between negatively charged membranes and the positively charged beads. The interaction is nonselective and therefore negatively charged ligands will bind to these beads.
- The binding capacity of WGA-PVT beads is typically 10-30 μg membrane protein per mg of bead
- The typical binding capacity of poly-L-lysine beads is 10 μg membrane protein per mg of bead
- Incubation time can often be reduced by agitating the assay tubes or plates. Agitation is desirable for assays employing WGA-PVT beads. Agitation is essential for assays employing Poly-L-lysine beads.
- The rate of association of membranes with WGA-PVT and PL-YS beads is relatively rapid (20-60 minutes).
Q. What receptor sources are suitable for use in receptor-binding SPA assays?
A. Whole cells, membrane preparations, and purified or soluble receptors can be used in SPA assays. The coupling of the receptor source to the beads should be considered carefully. For example, wheat germ agglutinin coated SPA beads would not be appropriate for coupling to cytosolic receptors, nuclear or other non-membrane bound receptors.
Q. What concentration of SPA bead and membrane should I use in a receptor binding SPA assay?
A. Perform an initial matrix experiment to determine the optimum amount of membrane and bead to use in the assay. The following guidelines should help you optimize the ratio of membrane and bead used in your assay:
- Use constant radioligand concentration and constant volume
- Include a "no membrane" control at each concentration of bead tested to observe NSB directly to bead.
- Set up B0 and NSB tubes at each combination of membrane and bead to be tested. For iodinated ligands, titrate from 0.25 - 2 mg SPA bead per well and 5 μg - 50 μg membrane protein per each bead concentration. For tritiated ligands, titrate 0.5 - 4 mg SPA bead per well and 5 μg - 100 μg membrane protein.
- For pre-coupled bead assays, the procedure of optimizing the membrane to bead involves pre-coupling increasing amounts of membrane to a fixed amount of bead, then testing a fixed aliquot (0.5 mg or 1 mg) in an assay with a fixed amount of radioligand to determine total and non-specific binding.
- Count the fully equilibrated assay and calculate specific counts bound and non-specific binding. Unlike filtration format assays, most receptor binding SPA assays require several hours to reach equilibrium.
- Plot the amount of membrane/receptor on the x-axis and specific binding counts on the y-axis for each bead amount assayed. Each bead concentration curve should reach a maximum count plateau, which is the point of bead saturation by the membrane. Examine the NSB counts for each curve and determine which amount of bead will yield the maximum signal-to-noise ratio.
Q. How do I couple cell membranes to SPA beads?
A. Cell membranes can be coupled to wheat germ agglutinin (WGA) coated SPA beads, and to poly-L-lysine beads. The wheat germ agglutinin binds to glycoproteins and glycolipids in the cell membrane. Poly-L-lysine binds to negative charged species in cell lysates. The binding capacity of WGA-coated beads is 10-30 μg membrane protein per milligram bead. The poly-L-lysine beads bind 10 μg membrane protein per mg bead, as it is less selective for cell membrane.
The membrane and bead can be added to the assay as separate, sequential additions (T=0 addition format), as a single reagent with the membrane bound to the bead (pre-coupled format), or the bead can be added to the assay after the membrane and ligand have equilibrated (delayed addition format). The ratio of membrane to bead has to be optimized to ensure that all the membrane present in the assay is coupled to SPA beads. Radioligand bound to uncoupled membrane will not contribute to the assay signal.
Q. Can I couple cell membranes to SPA beads before performing the assay?
A. The membrane can be pre-coupled to the SPA bead prior to adding the membrane or bead to the assay. The amount of membrane and bead needs to be optimized as part of the assay development. The procedure is to gently roll the membrane with the SPA bead for 1 to 4 hours at 2 - 8 degrees Celsius. The beads should then be pelleted by settling or gentle centrifugation at 1000 x g for 5 to 10 minutes at 4 degrees Celsius. The bead pellet should be washed twice in assay buffer to remove unbound cell membrane prior to resuspending in assay buffer to the working concentration.
Q. Can I couple the cell membrane to the bead after the ligand has bound to the receptor?
A. This assay format is described as delayed addition. The amount of bead added must be sufficient to bind all of the cell membrane in the assay well. The additional volume will cause the equilibrium of bound-to-free ligand to shift so the reaction must be given time to re-equilibrate. The membrane will bind to the beads in approximately 30 minutes. Excess bead does have to be added to ensure that all the membrane in the assay is coupled to bead.
Q. Do I need to remove unbound cell membrane from the assay?
A. It is essential that all membrane present in a receptor-biding SPA is bound to SPA bead. If radioligand is bound to membrane that is not coupled to SPA beads, then it will not contribute to the assay signal. For T=0 and delayed addition format, a slight excess of bead is added to ensure complete capture of the membrane. For pre-coupled bead format, it is essential to wash the beads after the pre-coupling stage to remove any unbound membrane.
Q. Do cell membranes detach from the bead?
A. Once bound the membrane should not detach from the bead surface, but the membrane or protease activity or bacterial contamination could degrade the receptor protein. The use of protease inhibitors and anti-bacterial agents may have to be considered for very long incubation times.
Q. What can interfere with the coupling of cell membrane to SPA beads?
A. In the case of WGA-coated beads, other glycosylated species might compete with N-acetyl-glucosamine resides for the WGA. These could be present in crude cell lysate preparations. If antibody-binding beads are being used, antibodies other than the specific anti-receptor antibody might be present in polyclonal antibody preparations. When using streptavidin beads with a biotinylated receptor, it is important that the receptor preparation is pure or non-receptor proteins could become biotinylated during the biotinylation reaction.
Q. Are the pH and ionic strength of the buffer important when coupling cell membrane to SPA bead?
A. The pH and ionic strength can be important depending on which coupling method is used. The antibody capture method may be more sensitive to pH and ionic strength. It should be remembered that the receptor protein will be affected by changes of pH.
Q. What time-of-addition format should I use to develop a receptor binding SPA assay?
A. There are three time-of-addition formats used for establishing a receptor binding assay, each with its own potential merits. In developing a receptor binding SPA, we generally recommend initially testing format conditions which closely mimic a published or existing assay format. In lieu of such information, it may be wise to investigate all three formats in parallel to determine the optimum condition which would produce the best achievable signal: noise ratio.
Refer to the section above for more information on these three formats.
| Method | Advantage |
|---|---|
| Membrane precoupled to SPA bead | May aid in lowering NSB |
| Time zero (T=0) addition of SPA beads | Easily automated |
| Delayed addition of SPA beads | Optimum ligand/receptor interaction possible |
Q. What are the advantages of the pre-coupled bead format?
A. The pre-coupled bead format reduces the number of additions made to the assay, as the beads and membranes are added as a single reagent. This can reduce the time to dispense an assay. The pre-coupling steps can help to reduce non-specific background counts. This is by ensuring that the bead surface is covered by cell membrane, so reducing the surface available for non-specific interaction of the radioligand with the bead. Also, because the membrane is coupled to the bead, there is no need to add excess bead to ensure a complete membrane capture. While adding excess bead does ensure that all the membrane is captured and maximum signal is obtained, the excess bead will contribute to a slightly increased non-specific binding and non-proximity effect.
Q. What are the advantages of the delayed addition format?
A. The addition of the SPA bead to the assay after the ligand has bound to the receptor avoids the bead interfering with the binding of the ligand to the receptor. This interference is only rarely observed. The incubation times for this format may be slightly shorter than T=0 addition formats. Once the receptor ligand assay has equilibrated, the beads are added and a further 30 - 40 minutes are required to ensure that the bead captures the membrane.
Q. What are the advantages of the T=0 addition format?
A. In the T=0 addition formats the ligand, receptor and bead are added as separate, sequential additions. The dispensing of reagents is simple, and the assay format is similar to a filtration format. As there is no requirement for processing the bead and membrane prior to the assay, reagents can be reconstituted or thawed on the day of the assay. It is also the easiest assay format with which to optimize the ratio of membrane to bead, as a matrix experiment can be set with increasing membrane and increasing bead. A slight excess of bead is required to ensure complete capture of the cell membrane present in the assay.
Q. Which format is most commonly used?
A. The T=0 addition format would appear to be the most used format because of the simplicity of dispensing.
Q. Will I get the same results with the T=0, delayed addition, and pre-coupled SPA bead receptor binding assay formats in an assay?
A. Unfortunately, there is no strict rule or generalization that can be applied to all assays. in some cases, there may be little or no difference in assay performance between formats, while in others there may be a format that yields better or worse assay windows. Therefore, it is always best to empirically determine this possibility. For example, with a radioligand that sticks to the bead surface, the pre-coupled bead format may help reduce the background by obscuring the bead surface from the radioligand. If binding of the receptor to the bead appears to interfere with the binding of ligand to receptor, then the delayed addition format may help as the bead is not present during the ligand-receptor binding event.
Q. What is the non-proximity effect (NPE)?
A. NPE occurs when either the concentration of the radioligand or the concentration of SPA beads is sufficiently high enough to elicit a signal from the emitted β-particles. This can occur even though the labeled ligand is not attached directly to the SPA bead through the interaction with the receptor or the non-specific interaction with the bead. In general, the signal is a linear function, directly proportional to the concentrations of each of these reagents. Therefore, a careful balance between radiolabel and SPA beads is crucial to maximize signal and sensitivity while minimizing NPE and ultimately cost. NPE effects show dilution dependence. The only technique available to minimize NPE is adjustment of the SPA bead or radiolabel concentrations.
Q. What is Non-specific binding (NSB) to SPA beads?
A. This signal is attributed to radiolabel which may adhere to the SPA beads themselves and not through a specific interaction with the receptor attached to the SPA bead. This component of background signal can be determined in the presence of an excess concentration of competitor in the absence of the membrane receptor. Reduction of this factor can be accomplished through the careful use of buffering systems and the appropriate bead type. Determination of the NSB to the SPA beads is separate from the NSB associated with membrane receptor preparations.
A competition experiment using an unlabeled compound in the absence or presence of added receptor may assist in identifying non-specific binding problems.
For routine SPA binding assays, non-specific binding may be a combination of non-specific binding to SPA beads as well as non-specific binding to the receptor and are expressed as one. Total non-specific binding is measured in the presence of an excess concentration of unlabeled competitor.
Citations
Using cell membranes
Chao, J. et al. C(4)-alkyl substituted furanyl cyclobutenediones as potent, orally bioavailable CXCR2 and CXCR1 receptor antagonists. Bioorg. Med. Chem. Lett 17, 3778-3783 (2007). Link
Mikami, T. et al. 5-Amino-6-chloro-N-[(1-isobutylpiperidin-4-yl)methyl]-2-methylimidazo[1,2-α]pyridine-8-carboxamide (CJ-033,466), a Novel and Selective 5-Hydroxytryptamine4 Receptor Partial Agonist: Pharmacological Profile in Vitro and Gastroprokinetic Effect in Conscious Dogs. Journal of Pharmacology and Experimental Therapeutics 325, 190-199 (2008). Link
Tzschentke, T.M. et al. (–)-(1R,2R)-3-(3-Dimethylamino-1-ethyl-2-methyl-propyl)-phenol Hydrochloride (Tapentadol HCl): a Novel μ-Opioid Receptor Agonist/Norepinephrine Reuptake Inhibitor with Broad-Spectrum Analgesic Properties. Journal of Pharmacology and Experimental Therapeutics 323, 265-276 (2007). Link
Wang, Y. et al. CCR2 and CXCR4 regulate peripheral blood monocyte pharmacodynamics and link to efficacy in experimental autoimmune encephalomyelitis. Journal of Inflammation 6, 32 (2009). Link
Using whole cells
Bettati, M. et al. Oxa-azaspiro derivatives: a novel class of triple re-uptake inhibitors. ChemMedChem 5, 361-366 (2010). Link
Allen, S.J., Ribeiro, S., Horuk, R. & Handel, T.M. Expression, purification and in vitro functional reconstitution of the chemokine receptor CCR1. Protein Expr. Purif. 66, 73-81 (2009). Link
Vaidehi, N., Pease, J.E. & Horuk, R. Modeling small molecule-compound binding to G-protein-coupled receptors. Meth. Enzymol. 460, 263-288 (2009). Link
Carrick, T. et al. Development of a scintillation proximity assay binding method for the human 5-hydroxytryptamine 6 receptor using intact cells. Anal. Biochem. 381, 27-32 (2008). Link
Iyer, C.V. et al. Rapid and recurrent neutrophil mobilization regulated by T134, a CXCR4 peptide antagonist. Exp. Hematol. 36, 1098-1109 (2008). Link
Other Revvity receptor binding assays
DELFIA fluorescent ligand-binding assays
Custom radiochemicals, SPA beads, and cell membranes
Revvity offers custom radiochemical, SPA beads, cell lines, cell membranes, plate barcoding, as well as custom assay development. If you are interested in custom services, please contact us.
Radiosynthesis and Labeling Custom Services
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.






































