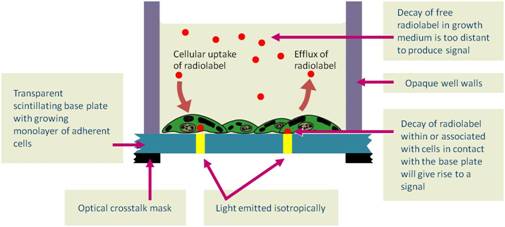
Overview
This plate-based version of scintillation proximity assays utilizes sterile, tissue-culture treated microplates. Cytostar-T™ scintillating microplates are sterile, tissue culture treated microplates designed not only for the growth of adherent but also suspension cell cultures. The integral, planar, transparent base of each well is composed of a proprietary homogeneous mixture of scintillants and polystyrene. The transparent nature of the base permits the observation of growth of cells plated in the well. Radioisotopes having suitable decay characteristics (3H, 14C, 35S, 45Ca, 125I) brought into proximity with the scintillant contained within the base (via radiochemical uptake or radiochemical interaction with the cells) will have that radioactive decay converted to a light signal. The amount of light generated is proportional to the amount of radioisotope within, or associated with, the cells.
Cytostar-T scintillating microplates are designed for use in the real-time analysis of a wide spectrum of cell associated phenomena. In almost all respects these plates may be used in an identical manner, and for similar applications to, standard plasticware produced specifically for tissue culture.
Principle of Cytostar-T plates
Cytostar-T scintillating microplates are standard format 96- or 384-well, sterile, tissue culture-treated microplates. The integral planar, transparent base of each well is composed of a proprietary homogeneous mixture of polystyrene and scintillants that permits the cultivation and observation of adherent cell monolayers. Radiochemicals labeled with radioisotopes having suitable decay characteristics (3H, 14C, 35S, 45Ca, 125I) can be brought into proximity with the scintillant-containing base by virtue of the biological processes within the cells, thereby generating light. The signal can be detected and quantified using appropriate instrumentation. The amount of light generated is proportional to the amount of radioisotope within the cell. No scintillation cocktail is required, as the scintillant is already embedded within the base of the plate.
Cytostar-T scintillating microplates are designed for non-invasive quantitation in real-time analysis of a wide spectrum of biological reactions in cells under normal physiological conditions. Like the SPA bead-based approach, these plates provide high-volume, homogeneous (no-wash) radiometric assays. CytoStar-T scintillating microplates conform to SBS-standards in both the 96-well and 384-well formats. The emitted blue light signal can be detected and quantified using a PMT-based radiometric detection instrument such as the MicroBeta2™.
The plates are compatible with both DMSO and ethanol in dilute aqueous solutions (<5% (v/v)). The plates also incorporate a design feature in the lid that minimizes the evaporation that occurs during incubation. The lid has condensation rings that reduce the risk of well-to-well contamination and, at the same time, reduces evaporation from individual wells. Without this feature, evaporation from marginal wells would be sufficient to produce an “edge effect”.
The transparency of the scintillating base facilitates the observation of live cells throughout an assay. The cell monolayer is best observed using an inverted microscope using simple brightfield optics, but enhanced contrast systems will provide more morphological information. However, the presence of the scintillants in the base precludes the plates use in fluorescence microscopy and the plastic construction precludes the use of polarization optics.
The format of a Cytostar-T plate easily lends itself to a matrix of conditions and treatments. Once optimization is established, the format of a Cytostar-T scinitillating microplate is suitable and amenable to automation and applications in high throughput screening.

Figure 1. Principle of a Cytostar-T scintillating microplate.
Products and catalog numbers
| Microplate | Description | Number of plates | Catalog number |
|---|---|---|---|
|
Cytostar-T 96-well
|
Sterile, tissue culture treated | 5 pack | RPNQ0162 |
| Sterile, tissue culture treated | 100 pack | RPNQ0163 | |
|
Cytostar-T 384-well
|
Sterile, tissue culture treated | 5 pack | RPNQ0165 |
| Sterile, tissue culture treated | 50 pack | RPNQ0166 |
Critical parameters
The following points are critical to the optimal function of the plates and interpretation of results:
- A reproducible monolayer of cells that remains in contact with the base
- Optimized counting conditions
- Use of appropriate controls in every plate
Cell culture protocol
Cytostar-T scintillating microplates are, with the exception of the scintillating base, 96- or 384-well sterile, tissue-culture treated microplates, and as such may be used in similar circumstances of principle and application as other microplates designed for cell culture. All the precautions and procedures normally undertaken during tissue culture should, therefore, be applied when using Cytostar-T scintillating microplates.
Cell culture - Generic protocol for seeding plates followed by 24-hour incubation
The production of a monolayer of cells from a chosen cell line is critical to the optimal function of the plate. The monolayer should remain in contact with the base throughout all the experimental manipulations. It is also wise to minimize well to well variation in plate response due to inconsistent and variable seeding densities in wells. Seeding densities of cells will be application and cell type dependent. The following protocol has been successfully used for typical adherent cell lines and may be modified to suit the characteristics of other cell lines. The use of non-adherent cell lines or the necessary avoidance of trypsin will require the development of an optimized and appropriate method for cell immobilization and disaggregation.

Cytostar-T workflow
Applications
- Whole cell ligand-receptor binding
- Thymidine uptake
- Glucose uptake
Other applications
- Cell cycle studies
- Cell signaling
- In situ RNA detection
- Arachidonic acid release
- Ion flux measurements
- Cell adhesion
- Cell-cell
- Cell-extracellular matrix (ECM)
- Drug metabolism
- Drug uptake/efflux
- Multidrug resistance
- Cell motility
- Leucocyte diapedesis
- Leucocyte chemotaxis
Counting plates and instrumentation
Cytostar-T plates may be counted using Revvity’s MicroBeta liquid scintillation counter. Users of Cytostar-T scintillating microplates are referred to the instrument manuals for detailed operating instructions.
There are, however, issues specific to Cytostar- T scintillating microplates in the optimization of the counting protocols that are highlighted here for guidance:
- When counting Cytostar-T microplates on microplate scintillation counters it is critical that a white card backing tape (catalogue number 6005199) is inserted below the microplates. Due to the arrangement of the detectors and the clear base of Cytostar-T plates, without the white card a significant proportion of the light generated will not be detected. Inclusion of the white card will increase the sensitivity of the counter.
- Where possible users can improve the efficiency of detection by replacing the supplied lid with a self-adhesive seal. Cytostar-T microplates should not be counted ‘open’ due to the risk of contaminating the detectors.
Energy window setting
It is essential to adjust the window settings as the instrument default settings may not be optimized for Cytostar-T scintillating microplates. Instrument default settings are normally based on liquid scintillation counting or SPA bead applications. Optimized window settings ensure maximum sensitivity for the chosen isotope and reduced backgrounds for the unique circumstances encountered with each instrument.
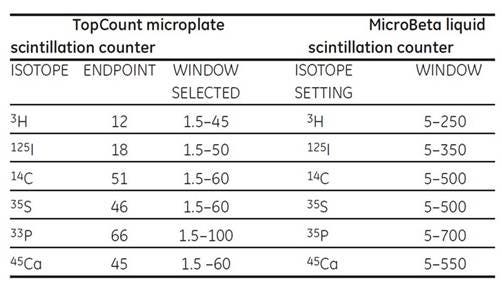
Table 1. Window settings for a TopCount microplate scintillation counter. This instrument determines the end point of the energy spectrum generated by each isotope.
Window settings for a MicroBeta liquid scintillation counter. Generation of an energy spectrum for the chosen isotope permits selection of maximum and minimum energy limits for inclusion in counting protocols.
Detector normalization
Normalization of the detectors should be undertaken for each instrument and isotope. The photomultiplier tubes may vary in detection efficiency and background contribution. The MicroBeta counter has a specific normalization procedure available for all isotopes. Where possible the performance of the detectors should be checked on a regular basis.
Quench correction
The mechanisms of quenching in Cytostar-T plates differ from those in traditional liquid scintillation counting due to the nature of the scintillant. The scintillant is effectively dissolved in the polymer base and is therefore unaffected by chemical quenching. Color quenching produced by the presence of compounds with the appropriate absorbance spectrum, between the point of origin of the photon and the detector, will reduce the counting efficiency of the plate. This is not normally a problem under conditions of constant quench. However, the overall efficiency of counting may be increased using phenol red-free media and colorless buffers, minimizing color quench. When using highly colored media that vary in intensity across a plate, a quench correction program should be utilized. This is especially important in high throughput applications that may necessitate the addition of a range of colored compounds to individual wells.
Correction for color quenched samples is performed using a standard curve constructed with a constant amount of radioactivity, a range of tartrazine (or other suitable colored reagent) solutions that will generate quenching over 0–90%, in a volume that reflects the assay conditions. For high energy isotopes such as 33P, 14C, 35S, two options are available to generate the standard curve:
- A solution of radioactivity with tartrazine can be used to generate the standard curve or,
- Cells labeled with the respective isotope, fixed to the plate, and a solution of tartrazine added to the wells can be used.
However, for low beta and beta-like energy isotopes (125I, 3H) the only option is to use labeled cells, as in “2”.
Crosstalk correction
Artifactual recording of counts in empty wells adjacent to those containing activity in Cytostar-T scintillating microplates may be a consequence of both optical and isotopic crosstalk. The provision of a printed mask on the base of the microplate will minimize the effect of optical crosstalk. Isotopic crosstalk counts are generated in adjacent wells by highly energetic particles. Isotopic crosstalk may be identified by generating the energy spectrum of the counts in the empty adjacent wells and corrected for using specific counting programs. For the isotopes commonly used in cell biology studies crosstalk is generally less than 5% and correction is not necessary, however 125I generates crosstalk at about 10%, due to the gamma component emitted during the decay event.
Solution background counts
Cytostar-T scintillating microplates will, by design of the scintillating base, detect most of the common beta emitting isotopes traditionally used in cell biology. When a solution containing a radiolabeled molecule is placed in the plate, counts are generated. These counts are referred to as solution background counts. The counts so generated are dependent upon the particle energy of the isotope and the distance the particle travels in an aqueous medium. Contributions from decay events at positions within the liquid contents of the well, that exceed the maximum particle distance for that isotope are not therefore recorded. The plate response to increasing radioactive concentration is linear.
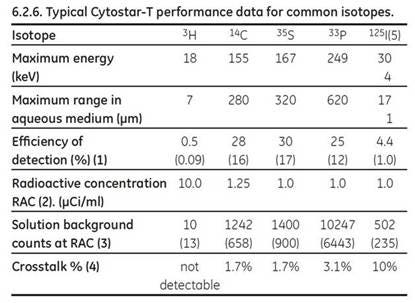
Table 2. Typical Cytostar-T scintillating microplate efficiency data for commonly used isotopes with applications in cell biology. Data generated on the MicroBeta counter. Figures in parentheses refer to values derived on the TopCount counter.
- The efficiency if calculated from the total counts from a well at maximal uptake expressed as a percentage of the liquid scintillation counts derived from the solubilized cells from that well. These figures are very much dependent on the cell type. Closely adherent cells that spread to the substratum are more efficient at bringing the isotope into proximity with the base.
- Radioactive concentration of the labeled compound used expressed in μCi/ml.
- The solution background counts are those counts generated by the medium plus labeled compound but without cells.
- Signal generated in adjacent wells in non-crosstalk corrected counting programs expressed as a percentage of the counts in the well containing an isotopic solution originating the crosstalk, data for MicroBeta only.
- Two mono-energetic electrons are produced by electron capture.
- The data contained in all tables has been generated under standard counting conditions, the use of alternative counting conditions such as PARALUX COUNTING MODE (MicroBeta) will generate volumes different from those above.
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























