Overview
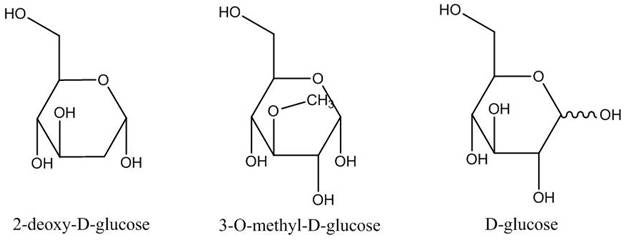
Glucose uptake experiments are commonly used to measure cellular metabolic activity and glucose transport. Glucose uptake can be studied using radiolabeled glucose itself, or radiolabeled glucose analogs such as 2-deoxy-D-glucose (DOG) or 3-O-methyl-D-glucose (OMG). The most common technique is use of radiolabeled 2-deoxy-D-glucose, a glucose analog. Once 2-deoxy-D-glucose has been taken up by cells, it is phosphorylated and cannot be metabolized further. Labeled 2-deoxy-D-glucose phosphate is trapped in the cell (unidirectional transport) reference. By contrast, 3-O-methyl-D-glucose is not phosphorylated and equilibrates across the cell membrane reference. Because equilibrium is usually reached rapidly, uptake is typically linear for only a short period of time (you may need to make your measurements quickly). D-glucose itself (as opposed to glucose analogs such as 2-deoxy-D-glucose or 3-O-methyl-D-glucose) can be incorporated into lipids, providing a measurement of glucose transport reference. Keep in mind, though, that D-glucose is a metabolized tracer.

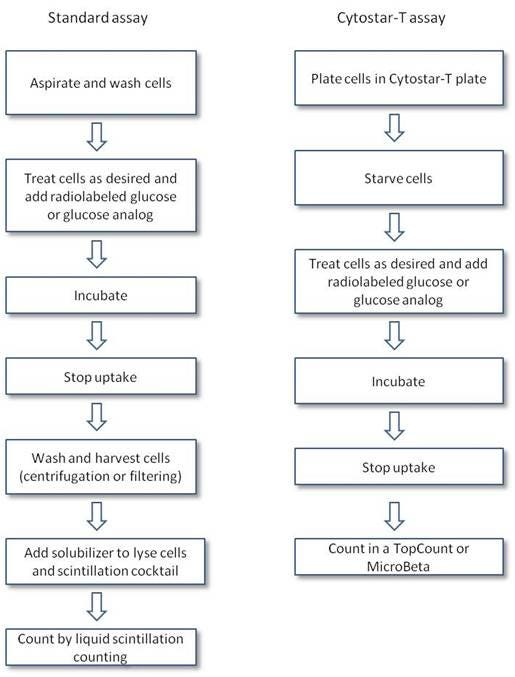
The assay is typically performed by feeding cells radiolabeled glucose or glucose analog, then separating radiochemical that has not been taken up by the cells by centrifuging or filtering (using a GF/C filter) the cells prior to adding scintillation cocktail and counting. Alternatively, the assay can be performed in a high-throughput format using Cytostar-T™ plates. These special tissue culture-treated plates can be used for adherent cell assays and contain scintillant embedded in the plastic base of the plate. If the radiolabeled glucose or glucose analog is taken up by the cells adhered to the bottom of the plate, this puts the radiolabel into proximity of the base of the plate where it can interact with the scintillant embedded in the plastic to generate signal. Radiolabeled glucose or analog that has not been taken up by the cells will be floating freely within the well and will not be able to interact with the scintillant embedded in the base of the plate.
What do I need to run this assay?
Standard low-throughput assay
- 3H- or 14C-labeled glucose or glucose analog (refer to table below)
- Unlabeled glucose or glucose analog stock
- Cells
- Culture medium, feeding buffer lacking glucose, PBS, etc. as necessary
- Tissue culture equipment, plates
- Test compounds, etc. as appropriate
- Tubes for centrifuging cells
- Scintillation vials
- Scintillation cocktail
- Liquid scintillation counter (we recommend a Tri-Carb™ liquid scintillation counter)
Standard filtration assay
- 3H- or 14C-labeled glucose or glucose analog (refer to table below)
- Unlabeled glucose or glucose analog stock
- Cells
- Culture medium, feeding buffer lacking glucose, PBS, etc. as necessary
- Tissue culture equipment, plates
- Test compounds, etc. as appropriate
- GF/C UniFilter plate for harvesting cells1
- Scintillation cocktail (we recommend MicroScint™-20 #6013621 if counting slightly damp plates, or Ultima Gold™ #6013326 if you are drying your plates prior to reading)
- BackSeal for plates1 (#6005199 - this will be used when you are going to add cocktail to the plate)
- TopSeal™-A for plates (#6050185)
- High-throughput radiometric detector (we recommend a MicroBeta™ detector)
-Alternatively, you can use GF/C filtermat with cassette and Meltilex™
High-throughput Cytostar-T assay
- 3H- or 14C-labeled glucose or glucose analog (refer to table below)
- Cytostar-T plates
- TopSeal-A™ adhesive plate seal (Cat. No. 6050185)
- White backseal, if counting from the top (Cat. No. 6005199)
- Unlabeled glucose or glucose analog stock
- Cells
- Culture medium, feeding buffer lacking glucose, PBS, etc. as necessary
- Tissue culture equipment
- High-throughput radiometric detector (we recommend a MicroBeta detector)
Products and catalog numbers
Radiochemicals
Refer to the information directly below this table for guidance on how to select a radiochemical for your assay.
| Chemical | Radioisotope | Labeling | Solvent | Specific activity | Rad. conc. | Storage | Cat. number |
| 2-deoxy-D-glucose | 3H | N (1,2 position) | 90% ethanol | 5-10 Ci/mmol | 1 mCi/mL | -20°C | NET328 |
| N (1,2 position) | steri-packaged aqueous solution | 5-10 Ci/mmol | 1 mCi/mL | 4°C | NET328A | ||
| N (1,2 position) | 90% ethanol | 25-50 Ci/mmol | 1 mCi/mL | -20°C | NET549 | ||
| 14C | (position 1) | 90% ethanol | 45-60 mCi/mmol | 0.1 mCi/mL | -20°C | NEC495 | |
| (position 1) | steri-packaged aqueous solution | 45-60 mCi/mmol | 0.1 mCi/mL | 4°C | NEC495A | ||
| U | steri-packaged aqueous solution | 250-350 mCi/mmol | 0.1 mCi/mL | 4°C | NEC720A | ||
| 3-0-methyl-D-glucose | 14C | (methyl group) | 90% ethanol | 30-60 mCi/mmol | 0.1 mCi/mL | -20°C | |
| D-glucose | 3H | N (2 position) | 90% ethanol | 20-30 Ci/mmol | 1 mCi/mL | -20°C | NET238C |
| (3 position) | 90% ethanol | 10 -20 Ci/mmol | 1 mCi/mL | -20°C | NET331C | ||
| (3 position) | steri-packaged aqueous solution | 10-20 Ci/mmol | 1 mCi/mL | 4°C | NET331A | ||
| N (5 position) | 90% ethanol | 10-20 Ci/mmol | 1 mCi/mL | -20°C | NET531 | ||
| N (6 position) | 90% ethanol | 25-50 Ci/mmol | 1 mCi/mL | -20°C | NET100C | ||
| 14C | (position 1) | 90% ethanol | 45-60 mCi/mmol | 0.1 mCi/mL | 4°C | ||
| (position 6) | 3% ethanol | 50-62 mCi/mmol | 0.2 mCi/mL | -20°C | NEC045X | ||
| U | 90% ethanol | 1-5 mCi/mmol | 0.1 mCi/mL | 4°C | |||
| U | 90% ethanol | 250-360 mCi/mmol | 1 mCi/mL | 4°C | |||
| U | 90% ethanol | 250-360 mCi/mmol | 0.1 mCi/mL | 4°C | NEC042X | ||
| U | 3% ethanol | 250-360 mCi/mmol | 0.2 mCi/mL | -20°C | NEC042V |
How to select a radiochemical from the above table:
- Chemical
Deoxy-D-glucose (DOG) is a glucose analog that is readily transported into most cells, is phosphorylated and trapped by the cells (unidirectional transport) and cannot be further metabolized. It is the most-common chemical used in glucose uptake assays.
3-O-methyl-D-glucose (OMG) is another glucose analog that is readily transported into most cells but does not become phosphorylated and therefore will equilibrate across the cell membrane. Equilibrium is usually reached rapidly, so the assay may only be linear for a short period of time; you may need to take your measurements quickly. This analog also cannot be further metabolized by cells.
D-Glucose (non-analog) can be incorporated into lipids, which provides a measurement of glucose transport. It is a tracer that is metabolized by the cells.
- Radioisotope: Either 14C-labeled glucose and glucose analogs, or 3H-labeled glucose and glucose analogs, can be used in glucose uptake assays. 14C has higher energy compared to 3H. 14C also has higher efficiency compared to 3H in liquid scintillation counting. Your radioactive license may restrict you to one radioisotope or the other. You will want to consult your radiation safety officer when selecting a radioisotope for your assay.
- Packaging
Glucose that is packaged in ethanol will be more-resistant to contamination by bacteria or other microbes. Preventing contamination is one way to ensure the longest shelf-life for a chemical (particularly in the case of glucose, because it is an excellent food-source for microbes).
For cell labeling or in vivo experiments, you may need to evaporate off any ethanol in the stock material prior to use, as ethanol can be toxic to cells.
Products described as being supplied as "Steri-packaged" are prepared with additional precautions to substantially reduce product bioburden and enhance product stability. Revvity makes no warranties, whether expressed or implied, with respect to the sterility or non-pyrogenicity of these or any products.
- Labeling positions: for uptake assays, the actual positioning of label is of less importance than it would be if you were performing an enzymatic assay, as uptake of the intact glucose or glucose analog is being traced.
U (uniformly labeled): designation for compounds labeled in all positions in a uniform or nearly uniform pattern
N (nominally labeled): designation when the method of preparation requires some (usually a significant amount) of the label to be at a specific site or sites, but no further information is available on the extent (if any at other positions)
G (generally labeled): designation for compounds in which there is a random distribution of radioactivity at various positions
Specifically labeled: designation used when all labeled positions are identified and the radioactivity at these positions is greater than 95% of the total incorporated into the compound
- Specific activity: the higher the specific activity, the greater the amount of radioactivity there is per glucose molecule. Specific activity is given in units of Curies per millimole of glucose or glucose analog, or milliCuries per millimole of glucose or glucose analog. The theoretical maximum specific activity of tritium is ~29 Ci/mmol (Curies per millimole of tritium). 3H products with a specific activity over this value indicate that on average, each glucose or glucose analog molecule contains more than one tritium. The theoretical maximum specific activity of 14C is ~62 mCi/mmol (milliCuries per millimole of carbon). 14C products with a specific activity over this value indicate that on average, each glucose or glucose analog molecule contains more than one labeled carbon. Specific activity can be decreased by adding cold glucose or glucose analog.
Scintillation cocktails
- Ultima Gold™ (Cat. No. 6013326): multipurpose liquid scintillation cocktail for aqueous and non-aqueous samples
- Ultima Gold MV (Cat. No. 6013151): can be used as scintillation cocktail for dry or wet filters including glass fiber, cellulose nitrate, cellulose acetate, mixed cellulose esters, nylon, and normal paper filters
- SOLVABLE™ (Cat. No. 6NE9100): solubilizer for lysing cells, compatible with Ultima Gold
- MicroScint-20 (Cat. No. 6013621): scintillation cocktail for counting slightly damp filters
Scintillation vials
- View our comprehensive list of available vials, caps, and assessories to suit your needs.
UniFilter plates
| Plate type | Filter type | Well format | Plates | Cat. number |
|---|---|---|---|---|
| UniFilter Plate | GF/C | 96-well | 50 | 6005174 |
Cytostar-T plates
| Product | Well format | Number of plates | Catalog number |
|---|---|---|---|
| Cytostar-T plates | 96-well | 5 | RPNQ0162 |
| 100 | RPNQ0163 | ||
| 384-well | 5 | RPNQ0165 | |
| 50 | RPNQ0166 |
Radiometric detectors
Protocol-in-brief
Tips
- Glucose uptake data are often expressed as moles of glucose per cell per unit time. To process your data, first convert from dpm (disintegrations per minute) to Curies using this equation:
1 Ci = 2.22 x 1012 dpm
- Once you have converted from dpm to Curies, you can use the specific activity of your lot of radiochemical to convert from Curies to moles. Make sure to multiply this result by the molar dilution factor (mol:mol) of cold glucose or glucose analog added to your assay.
Citations
Recommended citation: Tanti, J.F., Cormont, M., Grémeaux, T. & Le Marchand-Brustel, Y. Assays of glucose entry, glucose transporter amount, and translocation. Methods Mol. Biol 155, 157-165 (2001). Link
- Gay, R.J. & Amos, H. Counter-transport in chick embryo fibroblasts. A significant factor in measurement of glucose entry. Biochem. J 206, 301-309 (1982). Link
- Griffin, M.E. et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48, 1270-1274 (1999). Link
- Hajduch, E., Darakhshan, F. & Hundal, H.S. Fructose uptake in rat adipocytes: GLUT5 expression and the effects of streptozotocin-induced diabetes. Diabetologia 41, 821-828 (1998). Link
- Inoki, K., Haneda, M., Maeda, S., Koya, D. & Kikkawa, R. TGF-[bgr]1 stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells. Kidney Int 55, 1704-1712 (1999). Link
- Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest 115, 1627-1635 (2005). Link
- Pérez, A. et al. Endofacial competitive inhibition of the glucose transporter 1 activity by gossypol. Am. J. Physiol., Cell Physiol 297, C86-93 (2009). Link
- Rivas, C.I. et al. Increased Uptake and Accumulation of Vitamin C in Human Immunodeficiency Virus 1-infected Hematopoietic Cell Lines. Journal of Biological Chemistry 272, 5814 -5820 (1997). Link
- Tanti, J.F., Cormont, M., Grémeaux, T. & Le Marchand-Brustel, Y. Assays of glucose entry, glucose transporter amount, and translocation. Methods Mol. Biol 155, 157-165 (2001). Link
- Vera, J.C., Rivas, C.I., Zhang, R.H., Farber, C.M. & Golde, D.W. Human HL-60 myeloid leukemia cells transport dehydroascorbic acid via the glucose transporters and accumulate reduced ascorbic acid. Blood 84, 1628-1634 (1994). Link
- Wong, H.Y. et al. The effects of phenytoin and its metabolite 5-(4-hydroxyphenyl)-5-phenylhydantoin on cellular glucose transport. Life Sci 76, 1859-1872 (2005). Link
- Yin, J., Gao, Z., Liu, D., Liu, Z. & Ye, J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab 294, E148-156 (2008). Link
Custom services at Revvity
Revvity offers custom radiochemical (including GMP-certified radiochemicals), as well as other services. If you are interested in custom services, please contact us.
Radiosynthesis and Labeling Custom Services
For research only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























