

HTRF Human & Mouse Phospho-P38 MAPK (Thr180/Tyr182) Detection Kit, 10,000 Assay Points


HTRF Human & Mouse Phospho-P38 MAPK (Thr180/Tyr182) Detection Kit, 10,000 Assay Points






The phospho-p38 (Thr180/Tyr182) kit enables the cell-based quantitative detection of phosphorylated p38 as a readout of the MAPK pathway.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
The phospho-p38 (Thr180/Tyr182) kit enables the cell-based quantitative detection of phosphorylated p38 as a readout of the MAPK pathway.



HTRF Human & Mouse Phospho-P38 MAPK (Thr180/Tyr182) Detection Kit, 10,000 Assay Points



HTRF Human & Mouse Phospho-P38 MAPK (Thr180/Tyr182) Detection Kit, 10,000 Assay Points



Product information
Overview
This HTRF cell based assay conveniently and accurately quantifies phosphorylated p-38-MAPK at Thr180/Tyr182. This kit has applications in inflammation, immune response, and oncology research. Using a streamlined protocol, amenable to low-volume formats, this kit can be used from basic research to High Throughput drug screening.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Cardiovascular
Infectious Diseases
NASH/Fibrosis
Neuroscience
Oncology & Inflammation
|
| Unit Size |
10,000 Assay Points
|
Video gallery

HTRF Human & Mouse Phospho-P38 MAPK (Thr180/Tyr182) Detection Kit, 10,000 Assay Points

HTRF Human & Mouse Phospho-P38 MAPK (Thr180/Tyr182) Detection Kit, 10,000 Assay Points

How it works
Phospho-p38 MAPK (Thr180/Tyr182) assay principle
The Phospho-p38 MAPK (Thr180/Tyr182) assay measures p38 MAPK when phosphorylated at Thr180/Tyr182. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The Phospho-p38 MAPK (Thr180/Tyr182) assay uses 2 labeled antibodies: one with a donor fluorophore, the other one with an acceptor. The first antibody is selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the proteins phosphorylation state under a no-wash assay format.

Phospho-p38 MAPK (Thr180/Tyr182) 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding phospho-p38 MAPK (Thr180/Tyr182) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-p38 MAPK (Thr180/Tyr182) 1-plate assay protocol
Detection of Phosphorylated p38 MAPK (Thr180/Tyr182) with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
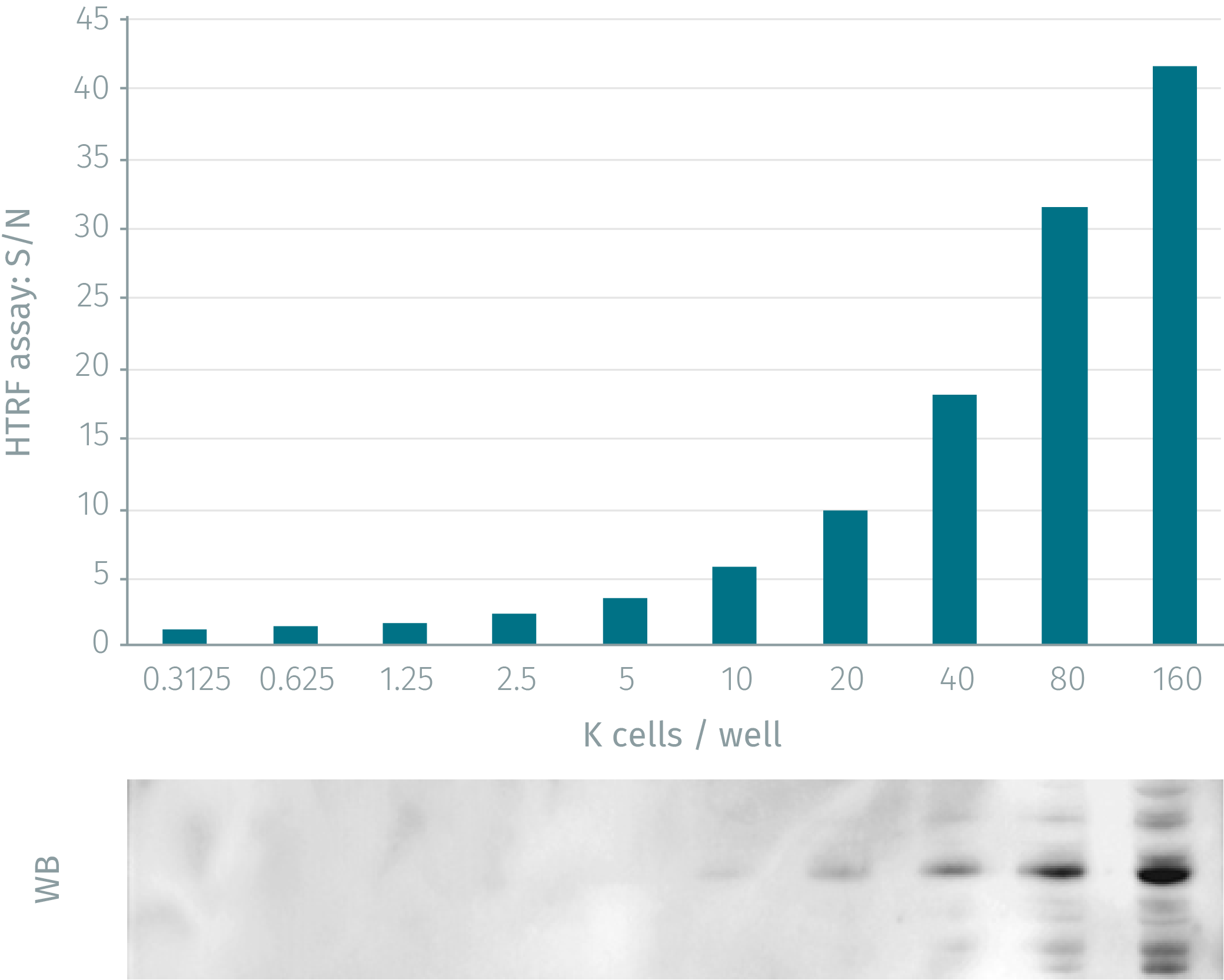
HTRF assay compared to WB using phospho-p38 MAPK cellular assay
HeLa cells were grown in a T175 flask 37°C, 5% Co2, 2 days. Stimulation was done with anisomycin 10µM for 15min. After elimination of cell culture medium, 3ml of supplemented lysis buffer were added and incubated for 30min. Soluble supernatants were collected after 10min centrifuging. Equal amounts of lysates were used for a side by side comparison of WB and HTRF. HTRF assay shows better sensitivity than Western Blot: 2500 cells for HTRF compared to10,000 cells for WB.
Anisomycin dose-response on NIH 3T3 cells
Murine NIH 3T3 cells (50,000 cells/well) were stimulated for 45 minutes at 37°C with various concentrations of anisomycin. After a 30 minutes lysis incubation time, phosphorylated p38 MAPK was measured using the two-plate assay protocol.

Anisomycin dose-response on Hela cells
Various concentrations of HeLa cells (25, 50 and 100K cells/well) were incubated for 45 minutes at 37°C with various concentrations of anisomycin. After a 30 minutes lysis incubation time, phosphorylated p38 MAPK was measured using the two-plate assay protocol.

Simplified pathway
Phospho-p38 MAPK (Thr180/Tyr182) simplified pathway
p38 MAPK is activated by a variety of cellular stresses, including osmotic shock, inflammatory cytokines, lipopolysaccharides (LPS), UltraViolet light, and growth factors. It is involved in cell differentiation and apoptosis. Activated p38 MAPK has been shown to phosphorylate and activate MAPKAP kinase 2 as well as phosphorylate the transcription factors ATF-2 , Max , and MEF2 . p38 MAPK plays a critical role in inflammation, immune response, apoptosis, cell differentiation, cell-cycle regulation and tumorigenesis.

Resources
Are you looking for resources, click on the resource type to explore further.
This guide provides you an overview of HTRF applications in several therapeutic areas.


How can we help you?
We are here to answer your questions.






























