
HTRF Human and Mouse Phospho-RNA Pol II (Ser2) Detection Kit, 500 Assay Points

HTRF Human and Mouse Phospho-RNA Pol II (Ser2) Detection Kit, 500 Assay Points




This HTRF kit allows for the cell-based quantitative detection of RNA Pol II when phosphorylated at Ser2.
| Feature | Specification |
|---|---|
| Application | Protein Quantification |
| Sample Volume | 16 µL |
This HTRF kit allows for the cell-based quantitative detection of RNA Pol II when phosphorylated at Ser2.


HTRF Human and Mouse Phospho-RNA Pol II (Ser2) Detection Kit, 500 Assay Points


HTRF Human and Mouse Phospho-RNA Pol II (Ser2) Detection Kit, 500 Assay Points


Product information
Overview
RNA polymerase II (RNA Pol II) is one of the primary enzymes responsible for expressing protein-encoding genes and some small nuclear RNAs. The carboxy-terminal domain (CTD) of RNA Pol II undergoes a cycle of phosphorylation, catalyzed mainly by members of the cyclin-dependent kinase (CDK) family. RNA Pol II CTD phosphorylation allows it to temporally couple transcription with transcription-associated processes (binding specificity of the CTD for particular factors is determined by the site-specific phosphorylation/dephosphorylation events).
Specifications
| Application |
Protein Quantification
|
|---|---|
| Brand |
HTRF
|
| Buffer/Solvent |
Lysis Buffer 1
|
| Detection Modality |
HTRF
|
| Host Species |
Human
Mouse
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
RNA pol II
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Immuno-oncology
Inflammation
|
| Unit Size |
500 assay points
|
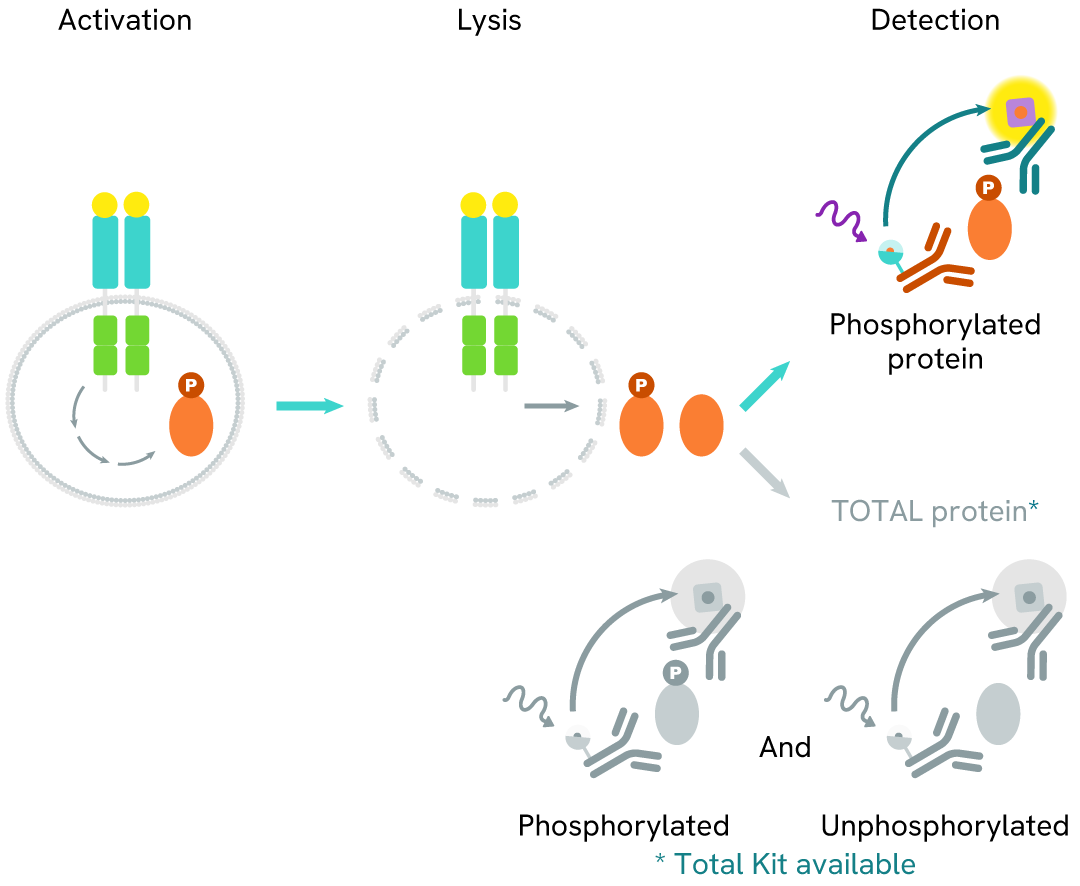
How it works
Phospho-RNA Pol II (Ser2) assay principle
The Phospho-RNA Pol II (Ser2) assay measures RNA Pol II when phosphorylated at Ser2. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The assay uses 2 antibodies, one labeled with a donor fluorophore and the other with an acceptor. The first antibody was selected for its specific binding to the phosphorylated motif on the protein, and the second for its ability to recognize the protein independently of its phosphorylation state. Protein phosphorylation leads to the formation of an immune-complex involving both labeled antibodies. This brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample and provides a means of assessing the protein's phosphorylation state under a no-wash assay format.

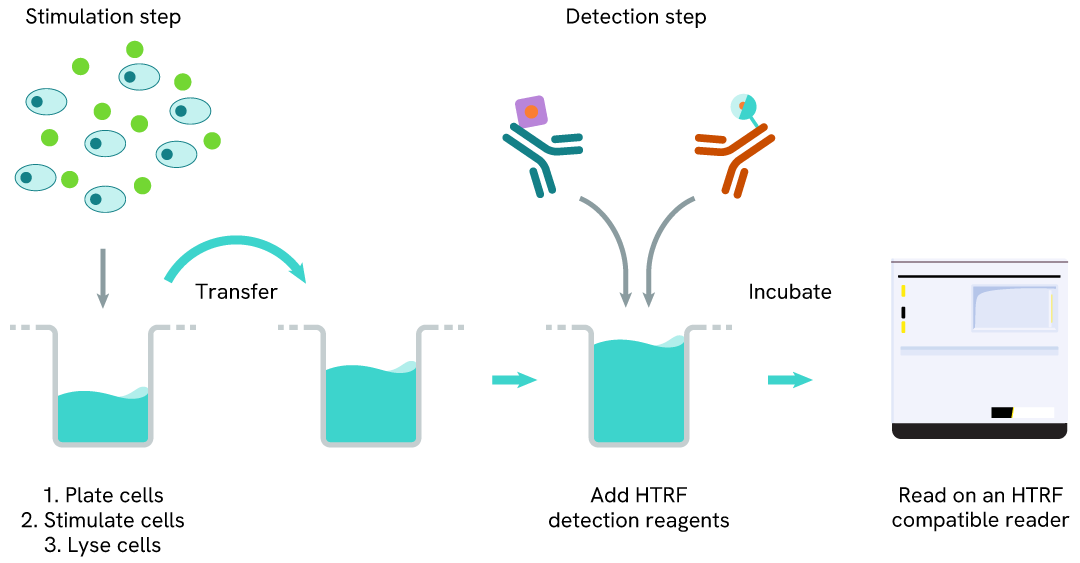
Phospho-RNA Pol II (Ser2) two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of RNA Pol II (Ser2) HTRF detection reagents. This protocol allows the cells' viability and confluence to be monitored.
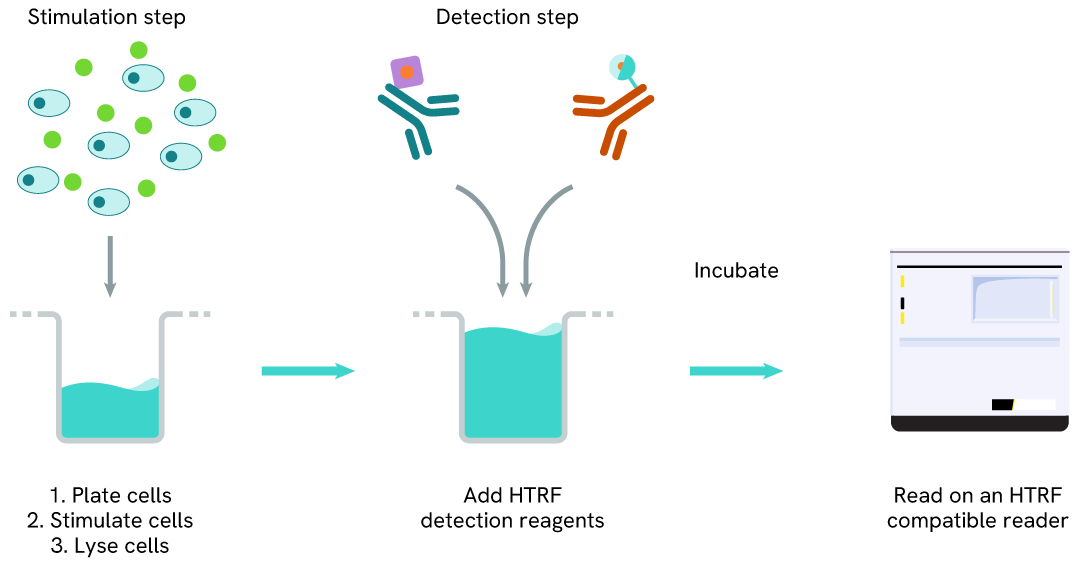
Phospho-RNA Pol II (Ser2) one-plate assay protocol
Detection of Phosphorylated RNA Pol II (Ser2) with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol allows for miniaturization while maintaining robust HTRF quality.
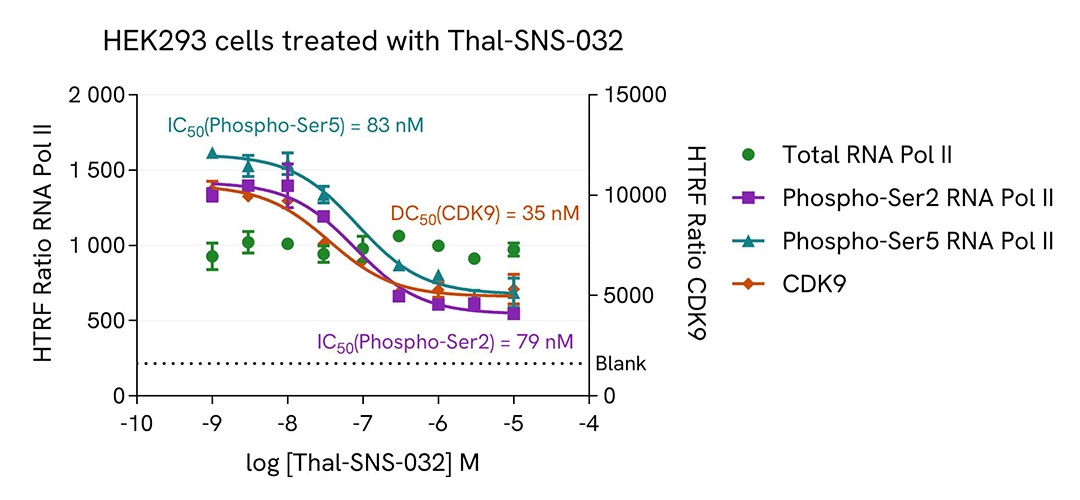
Assay validation
Inhibition of Phospho-Ser2 and -Ser5 RNA Pol II with Thal-SNS-032 in HEK293 cells
HEK293 cells were plated in a 96-well plate (50,000 cells/well) in complete culture medium, and incubated overnight at 37°C, 5% CO2. Then, cells were treated with a dose response of Thal-SNS-032, a selective CDK9 degrader, for 4 h. After culture medium removal, cells were lysed with 50 µL of supplemented Lysis Buffer #1 for 30 min at room temperature under gentle shaking. After cell lysis, 16 µL of cell lysate were transferred into a HTRF 384-well low volume white microplate then 4 µL of the HTRF Phospho-RNA Pol II (Ser2) or Phospho-RNA Pol II (Ser5) (Revvity, #64RNAP2S5PEG/PEH/PEY) or Total RNA Pol II (Revvity, #64RNAP2TPEG/PEH/PEY) or Total CDK9 (Revvity, #64CDK9TPEG/PEH/PEY) detection reagents were added. HTRF signal for four kits was recorded using HTRF compatible reader after an overnight-incubation at room temperature. In parallel, cell viability was assessed. To do this, 5 µL of the same cell lysate were transferred into an HTRF 96-well low volume white plate, and 25 µL of ATPlite 1step detection reagent were added (Revvity, #6016736/1/9). The luminescence signal was measured after a 10-min incubation in the dark at room temperature.
As expected, Thal-SNS-032, a selective CDK9 degrader, inhibited RNA Pol II phosphorylation on Serines 2 and 5 and decreased CDK9 expression level, without any significant effect on the expression level of the total RNA Pol II protein. In addition, this treatment did not induce cytotoxic effects, as measured by the cell viability indicator ATPLite (data not shown).
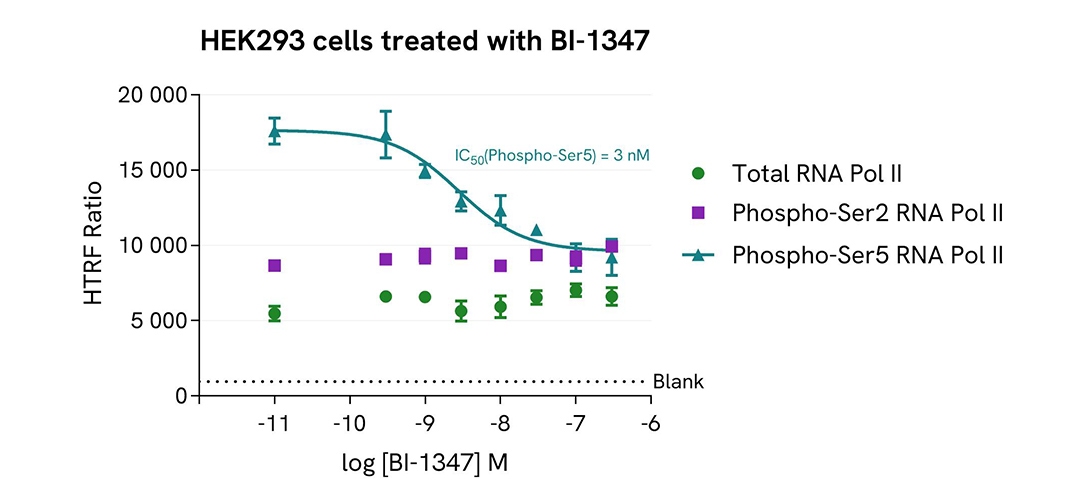
Inhibition of Phospho-Ser5 RNA Pol II with BI-1347 in HEK293 cells
HEK293 cells were plated in a 96-well plate (50,000 cells/well) in complete culture medium, and incubated overnight at 37°C, 5% CO2. Then, cells were treated with a dose response of BI-1347, a CDK8/cyclin C inhibitor, for 4 h. After culture medium removal, cells were lysed with 50 µL of supplemented Lysis Buffer #1 for 30 min at room temperature under gentle shaking. After cell lysis, 16 µL of cell lysate (neat for Total RNA Pol II and Phospho-RNA Pol II (Ser2) assays and dilution 1/4 for Phospho-RNA Pol II (Ser5) assay) were transferred into a HTRF 384-well low volume white microplate then 4 µL of the HTRF Phospho-RNA Pol II (Ser2) or Phospho-RNA Pol II (Ser5) (Revvity, #64RNAP2S5PEG/PEH/PEY) or Total RNA Pol II (Revvity, #64RNAP2TPEG/PEH/PEY) detection reagents were added. HTRF signal for three kits was recorded using an HTRF compatible reader after an overnight-incubation at room temperature. In parallel, cell viability was assessed. To do this, 5 µL of the same cell lysate were transferred into an HTRF 96-well low volume white plate, and 25 µL of ATPlite 1step detection reagent were added (Revvity, #6016736/1/9). The luminescence signal was measured after a 10-min incubation in the dark at room temperature.
As expected, BI-1347, a CDK8/cyclin C inhibitor, inhibited RNA Pol II phosphorylation on Serine 5, leading to a dose-dependent decrease of Phospho-RNA Pol II (Ser5) signal, without any significant effect on the expression level of the total RNA Pol II protein and phosphorylation level on Serine 2. In addition, this treatment did not induce cytotoxic effects, as measured by the cell viability indicator ATPLite (data not shown).
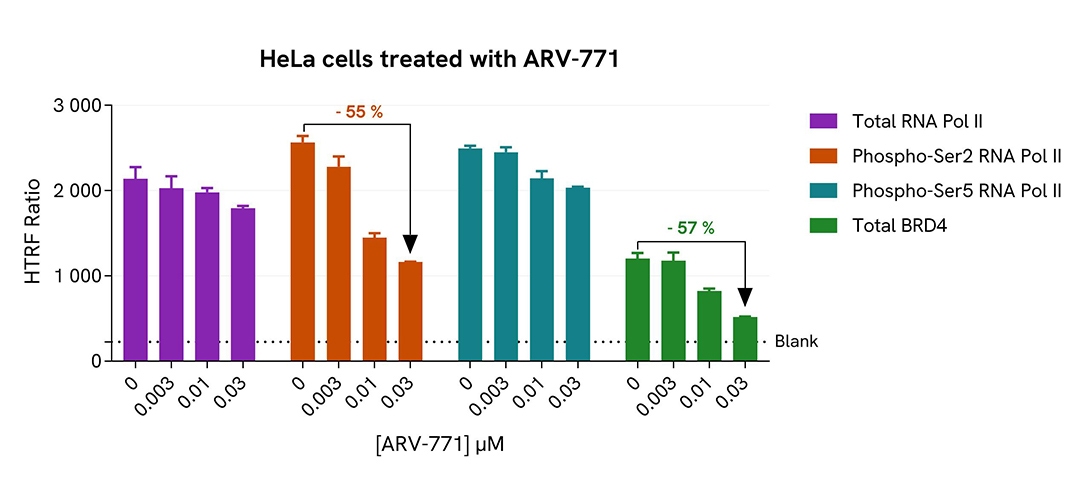
Inhibition of Phospho-Ser2 RNA Pol II with ARV-771 in HeLa cells
HeLa cells were plated in a 96-well plate (20,000 cells/well) in complete culture medium, and incubated overnight at 37°C, 5% CO2. Then, cells were treated with a dose response of ARV-771, a BRD4 degrader, for 20 h. After culture medium removal, cells were lysed with 50 µL of supplemented Lysis Buffer #1 for 30 min at room temperature under gentle shaking. After cell lysis, 16 µL of cell lysate were transferred into a HTRF 384-well low volume white microplate then 4 µL of the HTRF Phospho-RNA Pol II (Ser2) or Phospho-RNA Pol II (Ser5) (Revvity, #64RNAP2S5PEG/PEH/PEY) or Total RNA Pol II (Revvity, #64RNAP2TPEG/PEH/PEY) or Total BRD4 II (Revvity, #64BRD4TPEG/PEH/PEY) detection reagents were added. HTRF signal for four kits was recorded using Envision Nexus reader after an overnight-incubation at room temperature. In parallel, cell viability was assessed. To do this, 5 µL of the same cell lysate were transferred into an HTRF 96-well low volume white plate, and 25 µL of ATPlite 1step detection reagent were added (Revvity, #6016736/1/9). The luminescence signal was measured after a 10-min incubation in the dark at room temperature.
As expected, ARV-771, a BRD4 degrader, inhibited RNA Pol II phosphorylation on Serine 2 and decreased BRD4 expression level without any significant effect on the expression level of the total RNA Pol II protein and phosphorylation level on Serine 5. In addition, this treatment did not induce cytotoxic effects, as measured by the cell viability indicator ATPLite (data not shown).
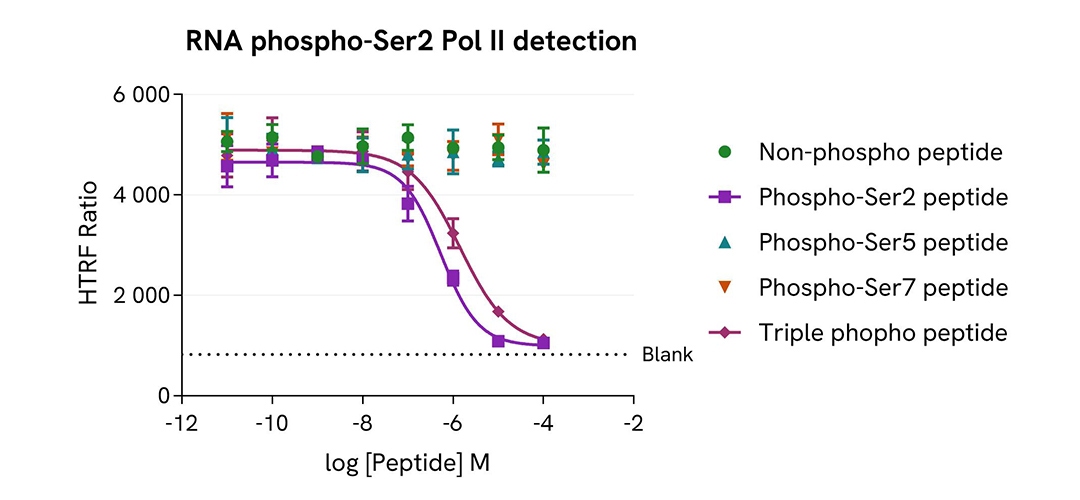
Selectivity of Phospho-Ser2 RNA Pol II assay using phospho-peptides competition
HeLa cells were cultured in flasks (4 millions / 175 cm2) in complete culture medium, and incubated 48 h at 37°C, 5% CO2. After culture medium removal, cells were lysed with 3 mL of supplemented Lysis Buffer #1 for 30 min at room temperature under gentle shaking. After cell lysis, 14 µL of cell lysate were transferred into a HTRF 384-well low volume white microplate then 2 µL of peptide (Non-phosphorylated or Phospho-Ser2 or Phospho-Ser5 or Phospho-Ser7 or Phospho-Ser2-Ser5-Ser7, named Triple phospho) and 4 µL of the HTRF Phospho-RNA Pol II (Ser2) detection reagents were added. HTRF signal was recorded using an HTRF compatible reader after an overnight-incubation at room temperature.
As expected, only Phospho-Ser2 and Triple phospho peptides led to a dose-dependent decrease of Phospho-RNA Pol II (Ser2) signal, demonstrating the specificity of the HTRF Phospho-RNA Pol II (Ser2) assay for the detection of the phosphorylation of Phospho-RNA Pol II on the specific residue Serine 2.
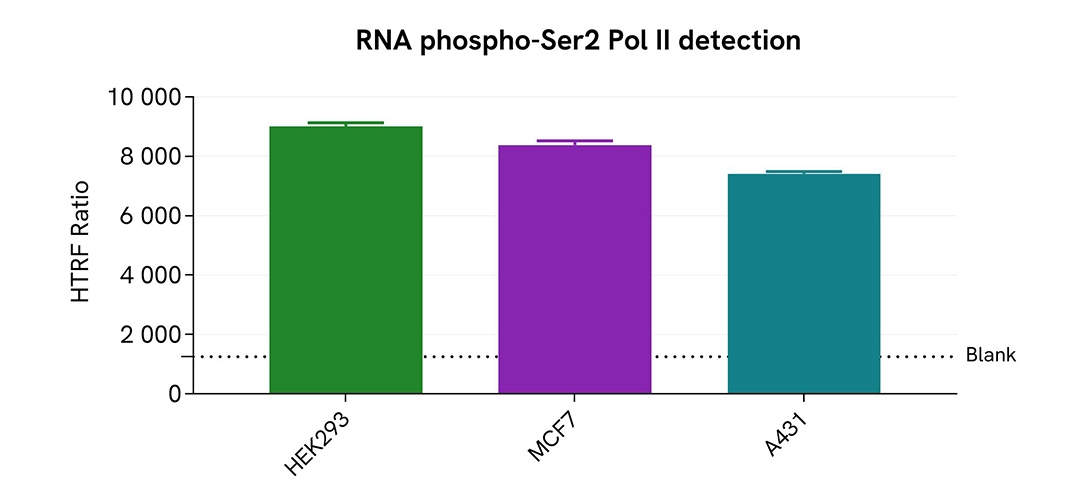
Assessment of Phospho-RNA Pol II (Ser2) level in various human cell lines
HEK293, MCF7 and A431 cells were cultured in flasks in complete culture medium, and incubated 48 h at 37°C, 5% CO2. After culture medium removal, cells were lysed with 3 mL of supplemented Lysis Buffer #1 for 30 min at room temperature under gentle shaking. After cell lysis, 16 µL of cell lysate were transferred into a HTRF 384-well low volume white microplate then 4 µL of the HTRF Phospho-RNA Pol II (Ser2) detection reagents were added. HTRF signal was recorded using HTRF compatible reader after an overnight-incubation at room temperature.
The HTRF Phospho-RNA Pol II (Ser2) assay detected RNA Pol II phosphorylation at residue Serine 2 in various cellular models with different phosphorylation levels.
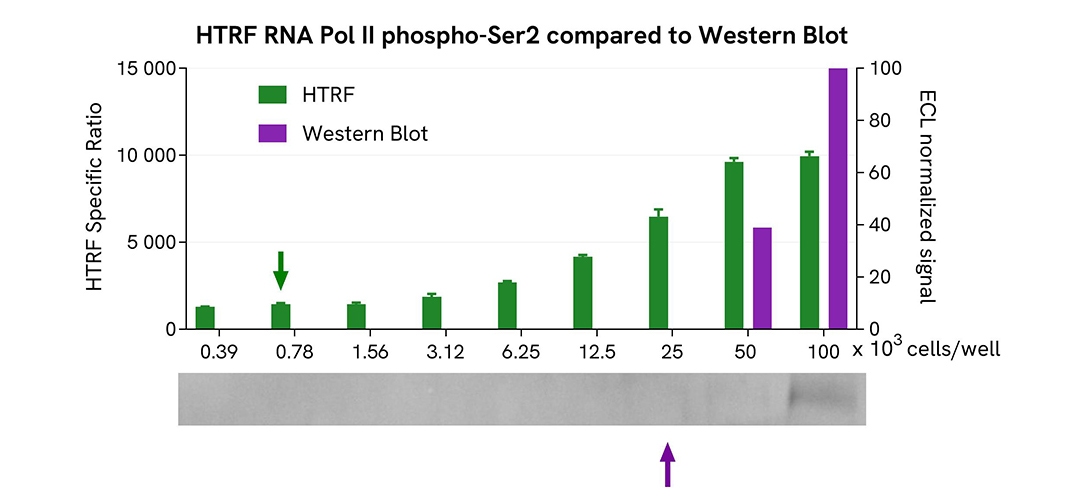
HTRF Phospho-RNA Pol II (Ser2) assay compared to Western Blot
HEK293 cells were cultured in flasks and incubated 48 h at 37°C, 5% CO2. After culture medium removal, cells were lysed with 3 mL of supplemented Lysis Buffer #1 for 30 min at room temperature under gentle shaking. Equal amounts of cell lysates (16 µL) were used for a side-by-side comparison of Western Blot and HTRF techniques.
Using the HTRF Phospho-RNA Pol II (Ser2) assay, 780 cells/well were enough to detect a significant signal, while more than 25,000 cells were needed to obtain a minimal chemiluminescent signal using Western Blot. Therefore, in these conditions, the HTRF Phospho-RNA Pol II (Ser2) assay is 32 times more sensitive than Western Blot technique.
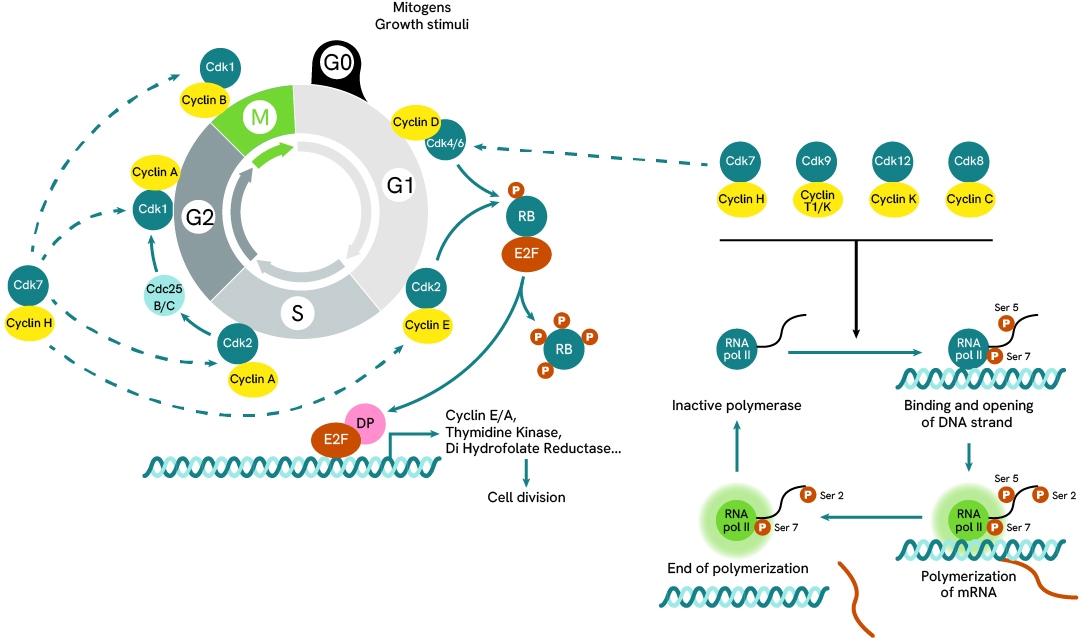
Simplified pathway
RNA Polymerase II signaling pathway
RNA Polymerase is under the regulation of CDKs such as CDK7 and CDK9, which are involved and expressed at different stage of the cellular cycle. RNA Polymerase’s role is to perform the transcription of DNA into mRNA, which it does by binding DNA promoter sequences and recruiting several partners to form a trancription complex capable of initiating the separation of the two DNA strands, RNA Pol II then moves along the strand and pieces together a matching mRNA strand from available nucleotides.
The activity of RNA Pol II is regulated by the phosphorylation state of its tail which can get sequentially phosphorylated at Ser7, Ser5 and Ser2. When unphosphorylated, the polymerase remains inactive, but when Ser7 and Ser5 become phosphorylatated they enable the assembly of the transcription complex. This is followed by the phosphorylation of Ser2 and RNA Pol II becoming fully active and mobile along the open DNA strand. As it performs transcription, RNA Pol II is progressively dephosphorylated at Ser5 then Ser7, and returns to an inactive state that detaches from the DNA strand,
The cycle of phosphorylation and desphosphorylation ensures the activation of RNA Pol II remains temporary and under control.
SDS, COAs, Manuals and more
Are you looking for technical documents related to the product? We have categorized them in dedicated sections below. Explore now.
- LanguageEnglishCountryUnited States
- LanguageFrenchCountryFrance
- LanguageGermanCountryGermany
- Resource TypeManualLanguageEnglishCountry-


Recently Viewed

How can we help you?
We are here to answer your questions.







































