
HTRF Human Total ALK Detection Kit, 500 Assay Points




The Total ALK kit is designed to monitor the expression level of cellular human ALK, and can be used as a normalization assay for the Phospho-human ALK Tyr1604 detection kit.
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption & disposal requirements under European REACH regulations (EC 1907/2006).
Product information
Overview
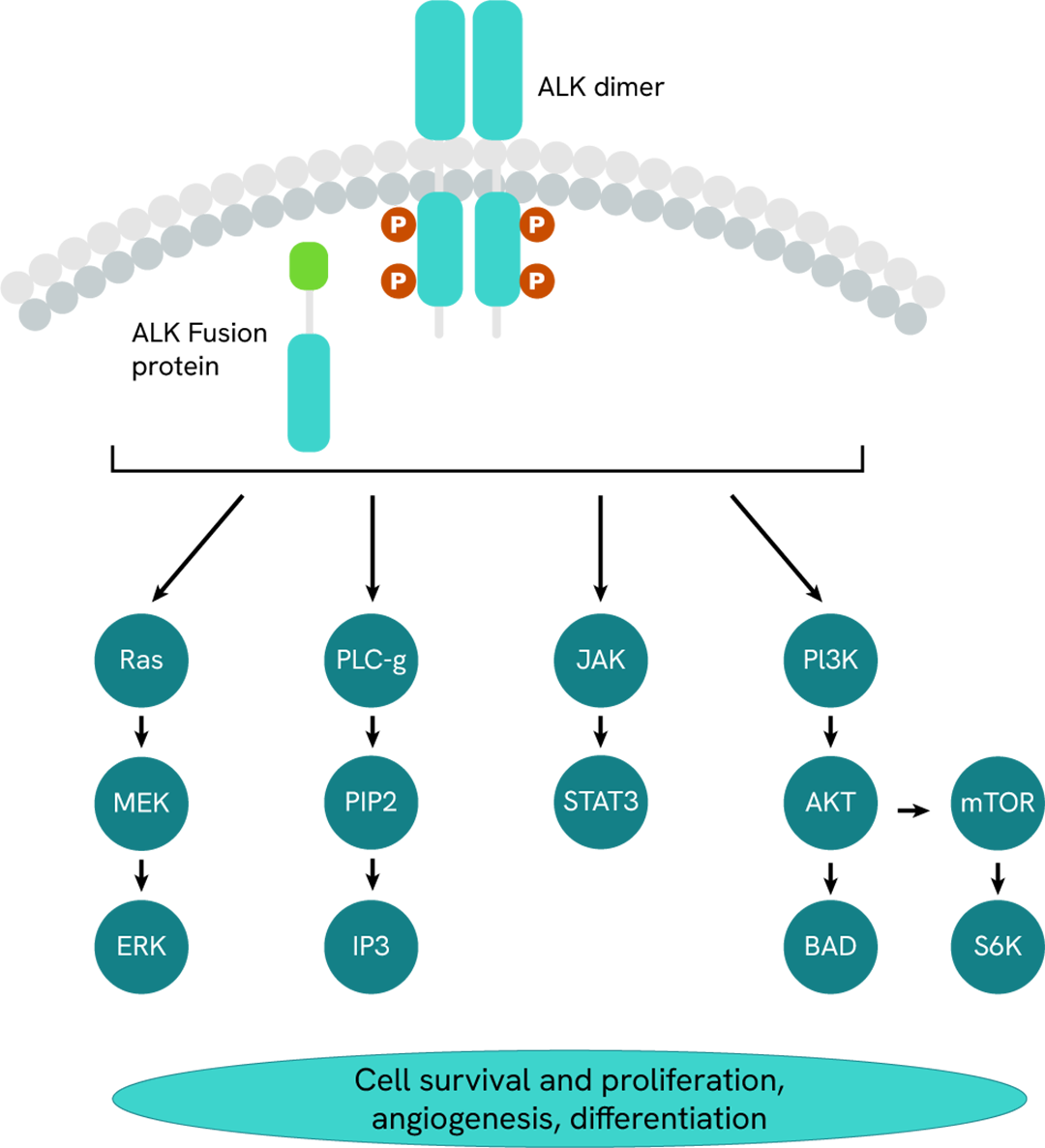
ALK (anaplastic lymphoma kinase) is a transmembrane tyrosine kinase belonging to the superfamily of insulin receptors. ALK regulates several pathways involved in cell survival, proliferation, and cell cycling, including the AKT/PI3K,MAPK/ERK, STAT3 and NFkB pathways.The ALK ligands, pleiotrophin (PTN) and midkine(MDK), induce ALK phosphorylation and activation on multiple Tyrosine including Y1604. ALK deregulation is associated with various cancers such as NSCLC, and neuroblastomas. Indeed, oncogenic ALK is driven by i) gene translocations and subsequent intracellular ALK fusion proteins (eg, EML4-ALK, NPM1-ALK, TPM3-ALK, VCL-ALK); ii) mutations (eg G263, R401, R551, P968 and E1242). Oncogenic ALK is constitutively active which results in uncontroled activation of downstream signaling pathways, leading to tumor cells survival and proliferation.
Specifications
| Brand |
HTRF
|
|---|---|
| Shipping Conditions |
Shipped in Dry Ice
|
| Unit Size |
500 Assay Points
|
How it works
Total ALK assay principle
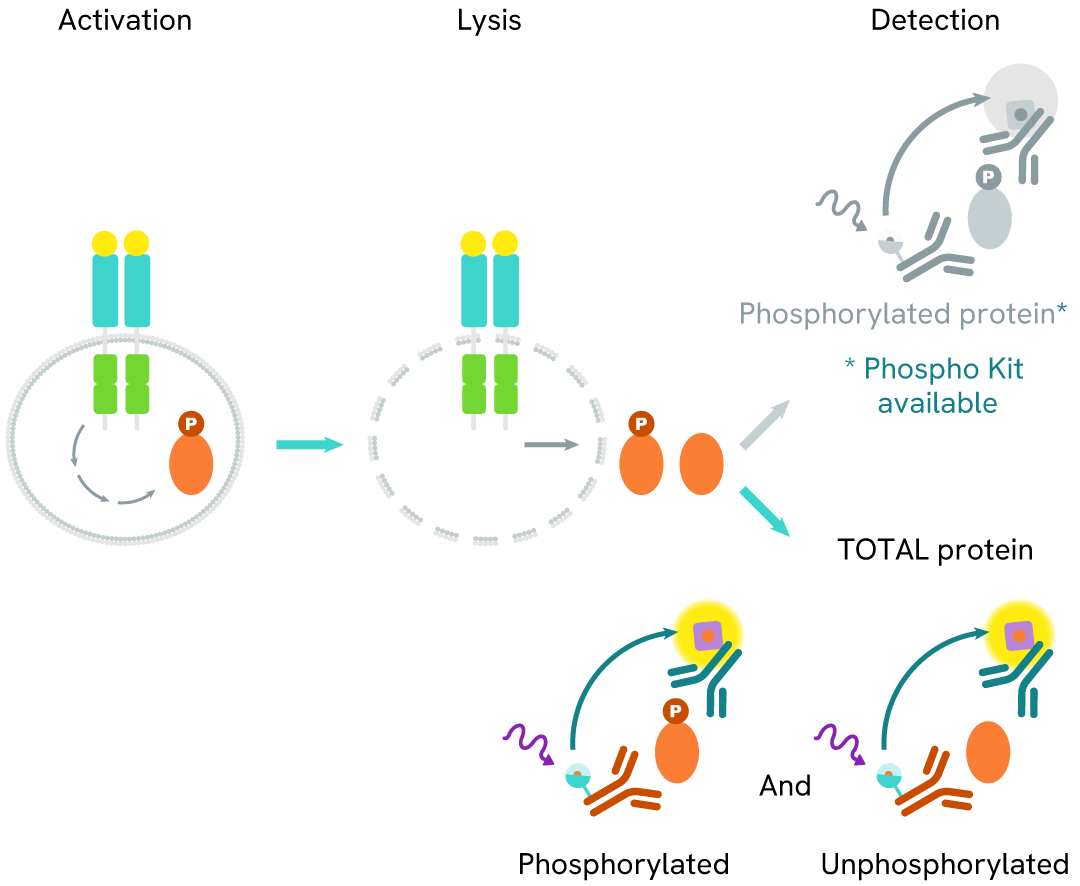
The Total-ALK assay quantifies the expression level of ALK in a cell lysate. Unlike Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis, or transfer. The Total-ALK assay uses two labeled antibodies: one coupled to a donor fluorophore, the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of ALK in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein’s expression under a no-wash assay format.

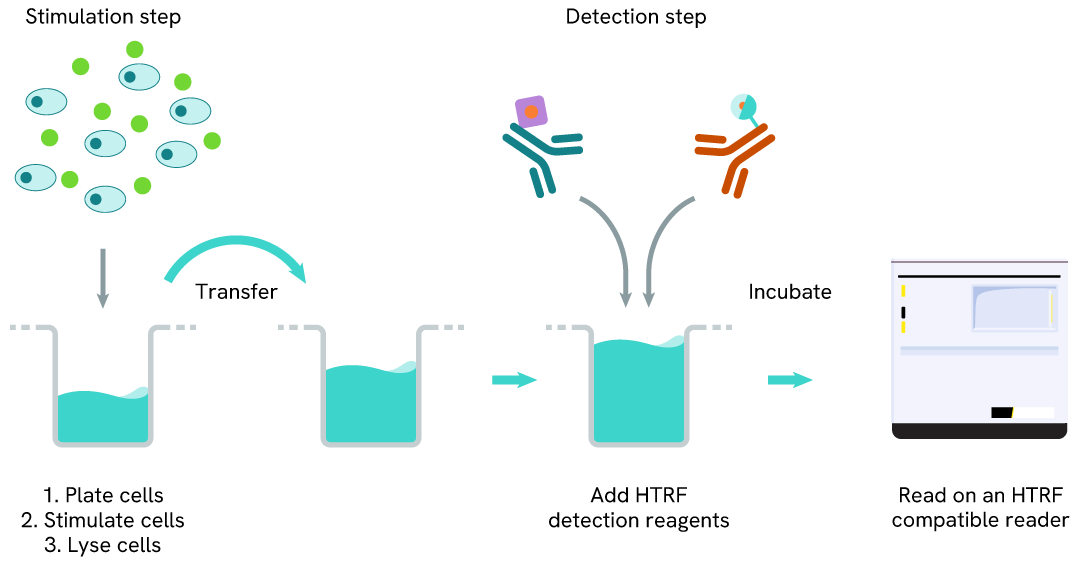
Total ALK two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a 384-well low volume detection plate before the addition of the Total-ALK HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.
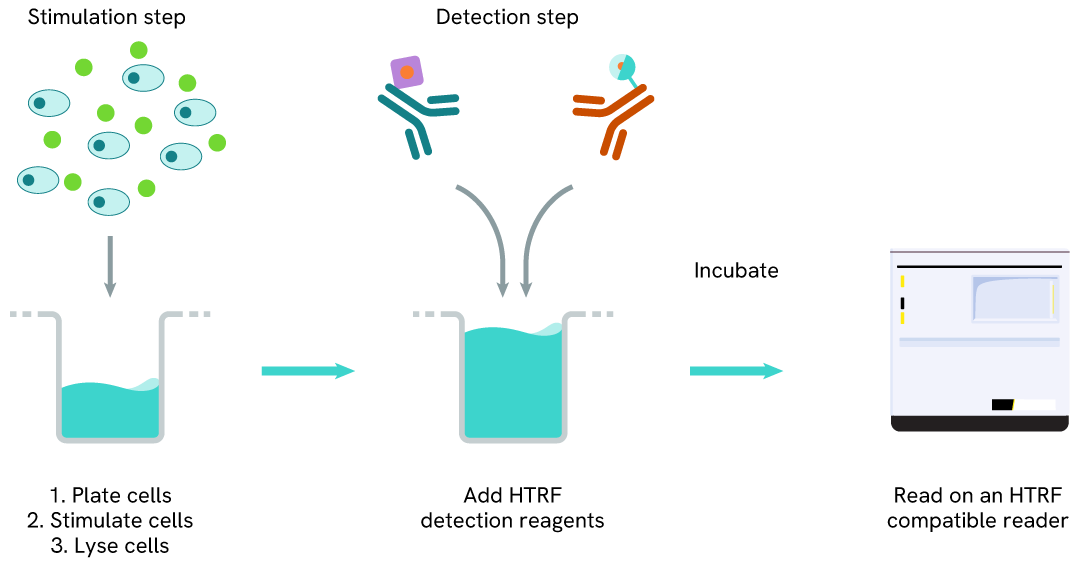
Total ALK one-plate assay protocol
Detection of Total ALK with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required.
This HTS-designed protocol enables miniaturization while maintaining robust HTRF quality.
Assay validation
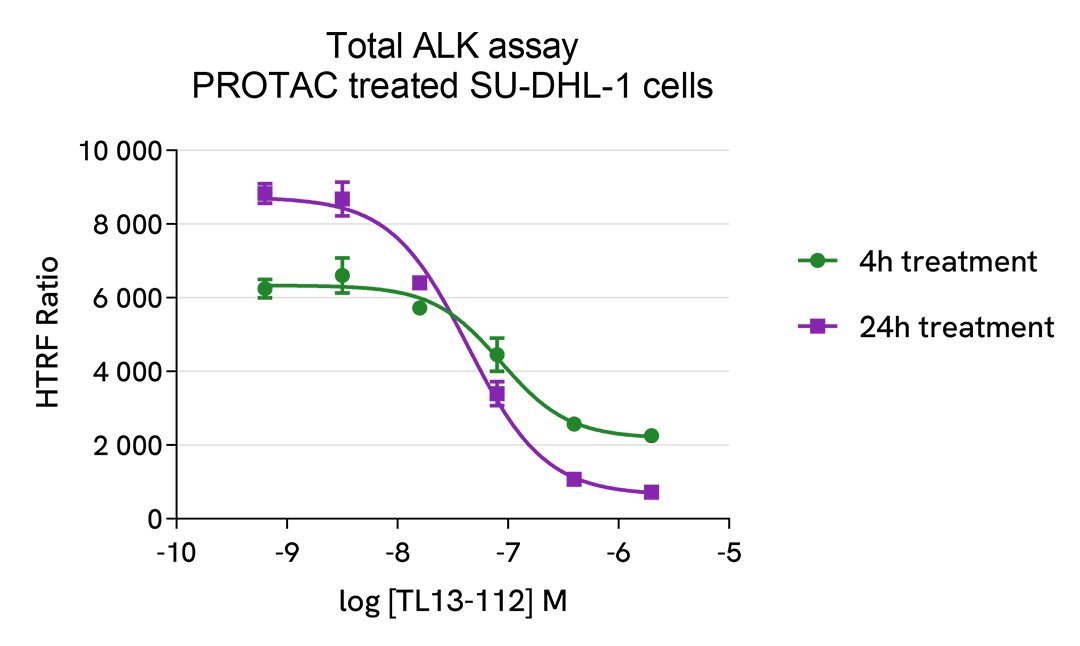
HTRF Total ALK modulation using PROTAC® on SU-DHL-1 cells
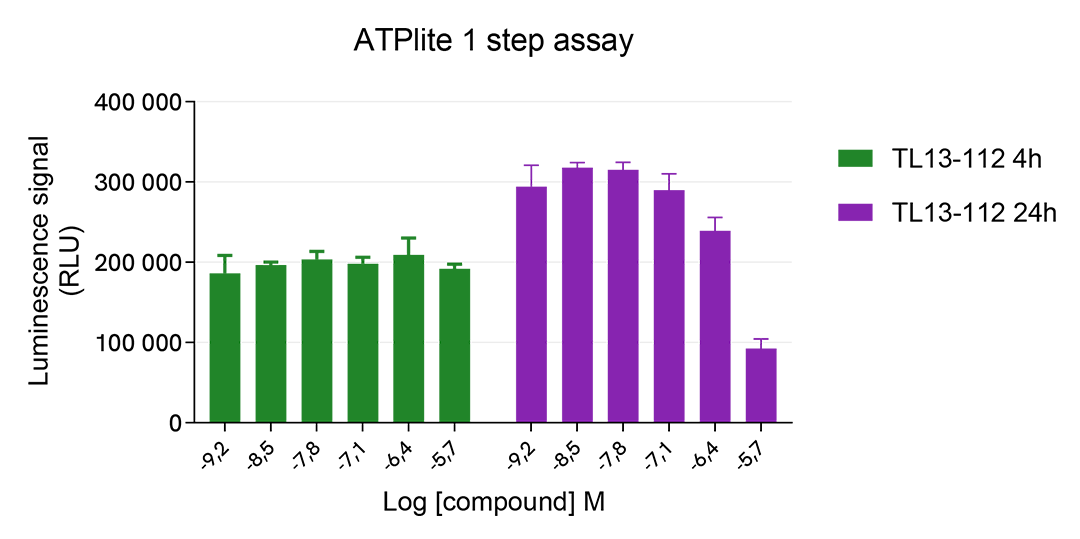
The experiments were performed using the two-plate assay protocol for suspension cells. SU-DHL-1 cells were plated in 96-well half area culture plates (10,000 cells/well) and treated with increasing concentrations of TL13-112 Protac, described as inducing ALK degradation.
After 24 hours of treatment at 37°C, 5% CO2, 10µL of supplemented Lysis Buffer#4 (4X) were dispensed into each well for 30min.
Then 16 µL of lysates were transferred into a ProxiPlate-384 (# 6008280/9), and 4 µL of the HTRF Total ALK detection antibodies were added. The HTRF signal was recorded after an overnight incubation at RT.
In parallel, cell viability was assessed. To do this, 5 µL of the same lysate were transferred into an HTRF 96-well low volume white plate (# 66PL96005/025/100), and 25 µL of ATPlite 1step detection reagent were added (# 6016736/1/9). The luminescence signal was measured after a 10-min incubation in the dark at RT.
TL13-112 PROTAC triggered a dose-dependent ALK protein decrease in the SU-DHL-1 cell model, which was more pronounced after 24h treatment. ATPLite results suggest cytotoxic effects associated with prolonged TL13-112 PROTAC treatment, as described in the literature.
HTRF Total ALK modulation using PROTAC® on SU-DHL-1 cells
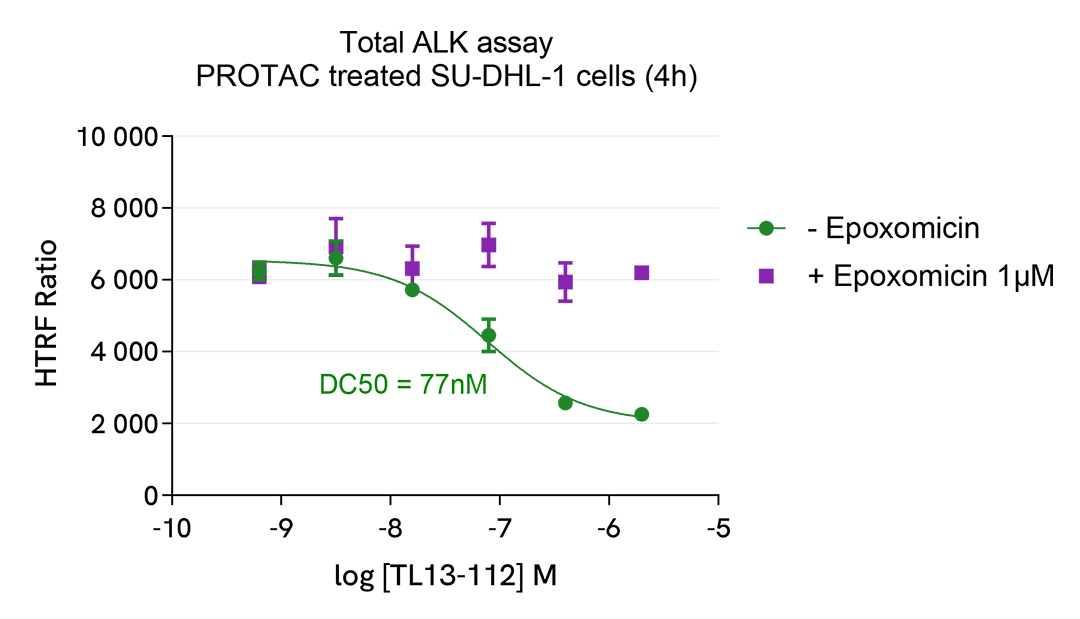
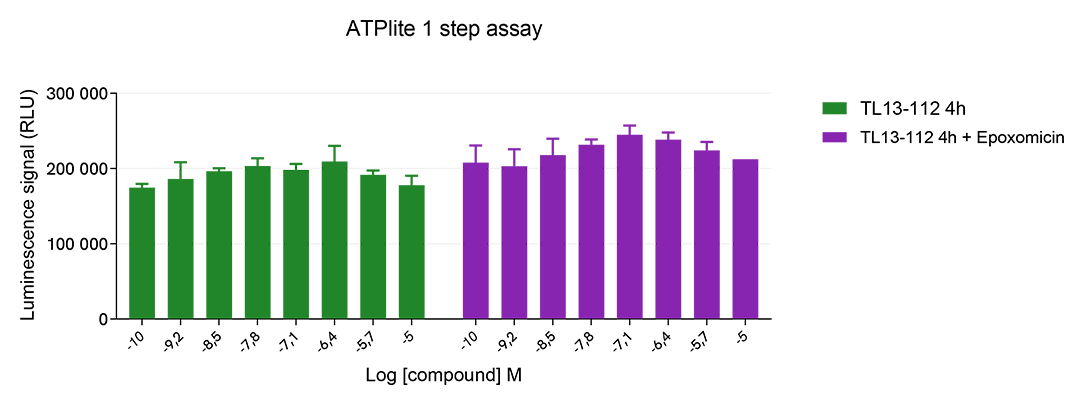
The experiment was performed using the two-plate assay protocol for suspension cells. SU-DHL-1 cells were plated in 96-well half area culture plates at a density of 10,000 cells/well, and treated with increasing concentrations of TL13-112 PROTAC, described as inducing ALK degradation. As a control, cells were pre-incubated with a proteasome inhibitor (Epoxomicin at 1µM) for 1h at 37°C prior to the addition of the PROTAC.
After 4 hours of treatment at 37°C, 5% CO2, 10µL of supplemented Lysis Buffer#4 (4X) were dispensed into each well.
Then 16 µL of lysates were transferred into a ProxiPlate-384 (# 6008280/9), and 4 µL of the HTRF Total ALK detection antibodies were added. The HTRF signal was recorded after an overnight incubation at RT.
In parallel, cell viability was assessed. To do this, 5 µL of the same lysate were transferred into an HTRF 96-well low volume white plate (# 66PL96005/025/100), and 25 µL of ATPlite 1step detection reagent were added (# 6016736/1/9). The luminescence signal was measured after a 10-min incubation in the dark at RT.
TL13-112 PROTAC triggered a dose-dependent decrease in ALK protein in the SU-DHL-1 cell model, whereas the expression level of ALK remained stable in the presence of the proteasome inhibitor epoxomicin. In addition, the compound did not induce cytotoxic effects, as measured by the cell viability indicator ATPLite. This result demonstrates that TL13-112 induced ALK degradation is mediated by the ubiquitin proteasome system.
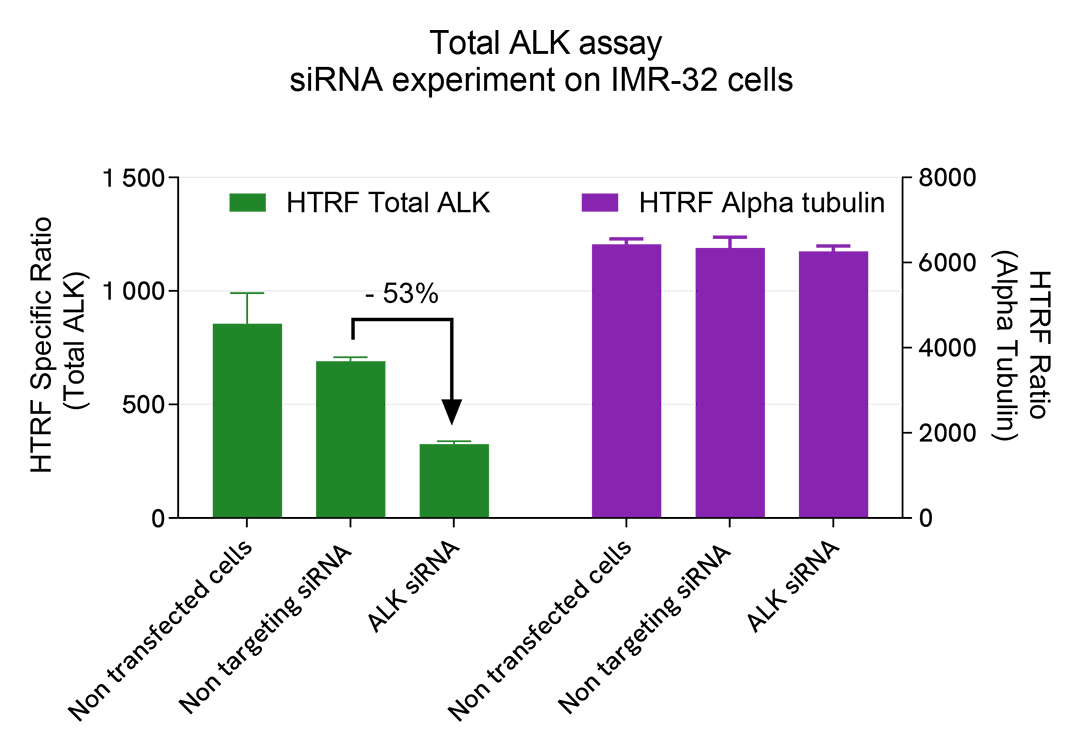
Specificity of HTRF Total ALK assay using siRNA
IMR-32 cells were plated in a 96-well culture plates at a density of 50,000 cells/well and incubated for 24 hours at 37°C, 5% CO2. The cells were then transfected with ON-TARGETplus SMARTPool siRNAs specific for ALK, as well as with a non-targeting siRNA as a negative control. After a 24h incubation with siRNAs, the medium was renewed for an additional 24h incubation and the cells were lysed with 50 µL of supplemented lysis buffer #4 for 30min.
Then 16 µL of lysates were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF Total ALK detection antibodies. An additional 4 µL of lysates (supplemented with 12 µL diluent #8) were also transferred into the microplate to monitor the alpha-tubulin level using the HTRF Alpha-tubulin Housekeeping Cellular Kit (64ATUBPET/G/H). The HTRF signal was recorded after an overnight incubation.
siRNA targeting ALK led to a 53% signal decrease compared to control cells transfected with the negative siRNA. The alpha tubulin level remained stable.
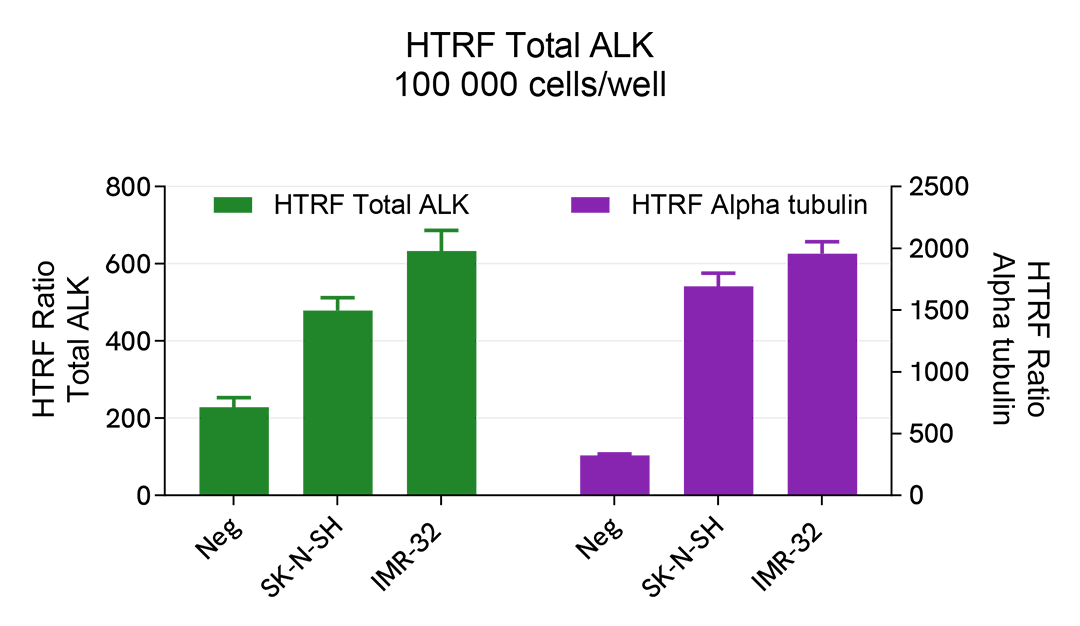
Total ALK assay versatility on human cell lines expressing wild type or mutated ALK
The experiments were carried out using the two-plate assay protocol for adherent cells. The human cell lines SK-N-SH expressing the F1174L mutation of the ALK protein and IMR32 expressing ALK wild type were seeded in a 96-well culture plate (100,000 cells/well in complete culture medium) and cultured at 37°C, 5% CO2 for 24 hours.
The day after, the cells were lysed with 50 µL of supplemented lysis buffer #4, and 16 µL of lysate were transferred into a ProxiPlate-384 Plus, 6008280/9) followed by the addition of 4 µL of HTRF Total ALK kit detection antibodies. In parallel, 4 µL of lysates (supplemented with 12 µL diluent #8) were transferred into the microplate, and alpha-tubulin housekeeping protein was detected with the HTRF Alpha-tubulin Housekeeping Cellular Kit (64ATUBPET/G/H). The HTRF signal was recorded after an overnight incubation.
The HTRF Total ALK kit efficiently detected ALK in various cell lines expressing WT ALK (e.g. IMR-32 cells), mutated ALK (eg SK-H-SH cells), or NPM-ALK fusion protein (e.g. SU-DHL-1 cells).
Simplified pathway
ALK (anaplastic lymphoma kinase) is a transmembrane tyrosine kinase which regulates cell survival, proliferation, and cell cycling, The ALK ligands, pleiotrophin (PTN) and midkine (MDK), induce ALK phosphorylation on multiple Tyrosine including Y1604 which activates multiple signaling pathways such as AKT/PI3K,MAPK/ERK, STAT3 and NFkB pathways.
Constitutive activation of oncogenic ALK fusion proteins or mutated ALK is associated with various cancers such as NSCLC, and neuroblastomas.
Resources
HTRF: unfamiliar territory?
This technical brochure reviews the general principles of HTRF™ and the associated Tag-lite™ technology...
Your guide for improving cell signaling assay performance
This complete Revvity guide provides you with all the tools you need to...
SDS, COAs, Manuals and more
Are you looking for technical documents for this product. We have housed them in a dedicated section., click on the links below to explore.


How can we help you?
We are here to answer your questions.






























