

HTRF Human & Mouse Phospho-c-Src (Tyr419) Detection Kit, 10,000 Assay Points


HTRF Human & Mouse Phospho-c-Src (Tyr419) Detection Kit, 10,000 Assay Points






The phospho-c-Src (Tyr419) kit is designed to monitor the autophosphorylation of c-Src on Tyrosine 419, which is one of the indicators of its activation.
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption and disposal requirements under European REACH regulations (EC 1907/2006).
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
The phospho-c-Src (Tyr419) kit is designed to monitor the autophosphorylation of c-Src on Tyrosine 419, which is one of the indicators of its activation.
For research use only. Not for use in diagnostic procedures. All products to be used in accordance with applicable laws and regulations including without limitation, consumption and disposal requirements under European REACH regulations (EC 1907/2006).



HTRF Human & Mouse Phospho-c-Src (Tyr419) Detection Kit, 10,000 Assay Points



HTRF Human & Mouse Phospho-c-Src (Tyr419) Detection Kit, 10,000 Assay Points



Product information
Overview
The Phospho-c-Src (Tyr419) assay is designed to monitor the expression level and the autophosphorylation of c-Src on Tyr419, which is one of the indicators of its activation and used in cancer research. c-Src is a member of the Src kinase family and shares a high degree of sequence identity with the ten other members. This protein is a membrane-associated, non-receptor tyrosine kinase. Its autophosphorylation on the residue Tyr419 indicates its full activation. c-Src kinase is over-expressed and highly activated in many human cancers.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 3
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Oncology & Inflammation
|
| Unit Size |
10,000 Assay Points
|
Video gallery

HTRF Human & Mouse Phospho-c-Src (Tyr419) Detection Kit, 10,000 Assay Points

HTRF Human & Mouse Phospho-c-Src (Tyr419) Detection Kit, 10,000 Assay Points

How it works
Phospho-c-Src Tyr(419) assay principle
The Phospho-c-Src Tyr(419) assay measures c-Src Ty when phosphorylated at 419. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The Phospho-c-Src Tyr(419) assay uses 2 labeled antibodies: one with a donor fluorophore, the other one with an acceptor. The first antibody is selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the proteins phosphorylation state under a no-wash assay format.

Phospho-c-Src-Tyr(419) 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding Phospho-c-Src-Tyr(419) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-c-Src-Tyr(419) 1-plate assay protocol
Detection of Phosphorylated c-Src-Tyr(419) with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
HTRF assays for c-Src validated by siRNA on HEK293 cells
Human HEK293 embryonic kidney cells were plated and cultured for 24h. Cells were then transfected with a c-Src siRNA or with a negative control and treated with increasing concentrations of H2O2 before medium removal and lysis. Lysates were transferred into a 384-well low volume white microplate and kit reagents were added. Cell treatment with the c-Src siRNA led to a huge decrease of the HTRF signal (~ 75%) compared to the cells transfected with the negative control siRNA, demonstrating that both kits are selective for c-Src and do not cross-react with other Src family members.

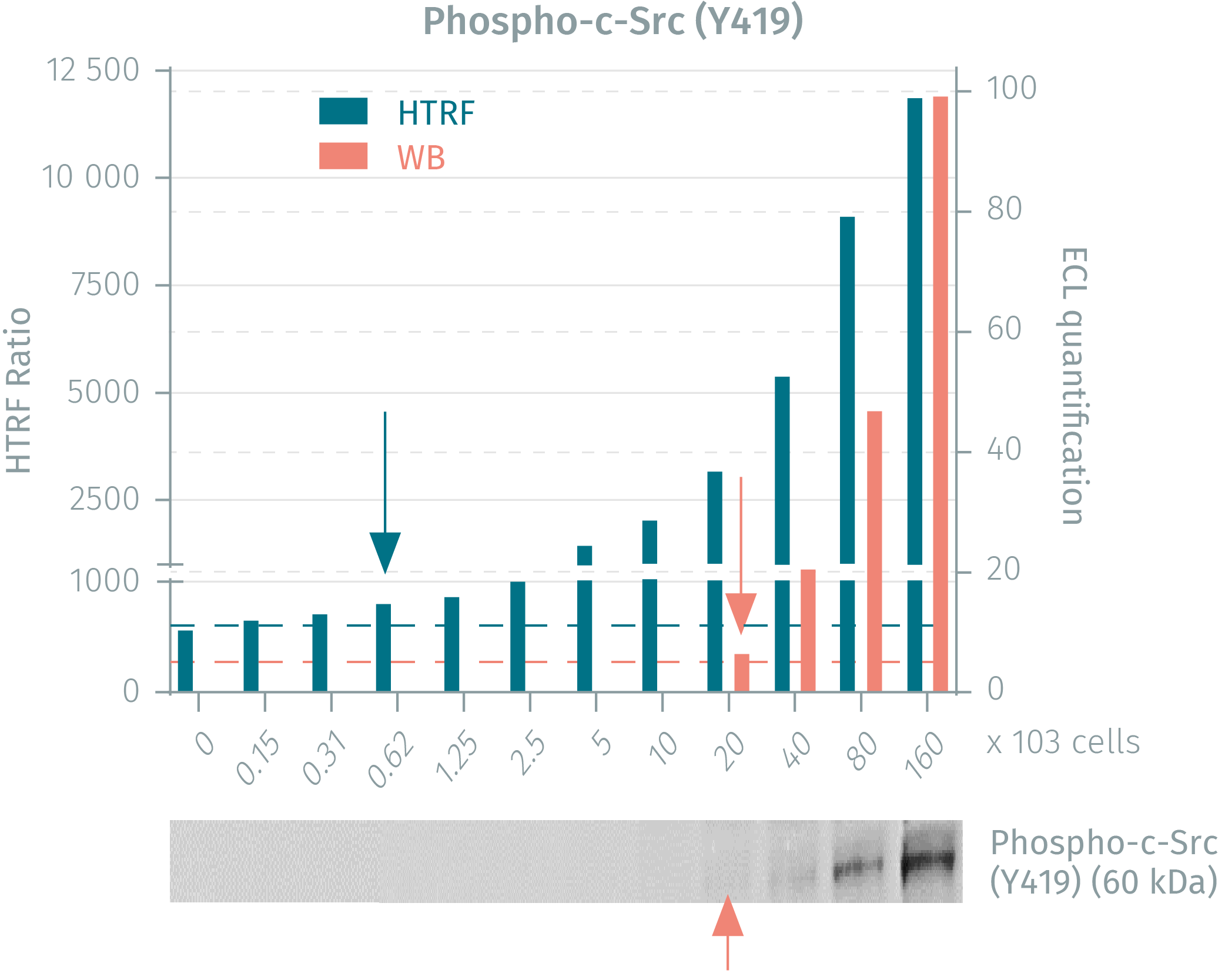
HTRF vs WB using phospho-c-Src assays on A431 cancer cells
Human A431 cancer cells were seeded in a T175 flask and cultured until 80% confluency was reached. Following cell lysis, soluble supernatants were collected via centrifugation and serial dilutions were performed in the supplemented lysis buffer and transferred into a 384-well low volume white microplate before finally adding HTRF phospho or total detection reagents. Equal amounts of lysates were used for a side by side comparison between Western Blot and HTRF. The HTRF phospho-c-Src assay is 32-fold more sensitive than WB while the total c-Src assay is 8-fold more sensitive than WB.
Bosutinib Inhibition of c-Src activity in HEK293 cells
HEK293 cells were plated at 100,000 cells/well in a 96-well plate, and incubated for 24h at 37°C, 5% CO2. After treatment for 30 minutes with increasing concentrations of Bosutinib and then stimulation with 20 mm H2O2 for 10 minutes, the medium was removed and the cells were lysed with 50 µL of supplemented lysis buffer #3 for 30 minutes at RT under gentle shaking. 16 µL of lysate were transferred into a 384-well low volume white microplate and 4 µL of the HTRF phospho- or total c-Src detection reagents were added. The HTRF signal was recorded after 4 hours of incubation.

c-Src activation in human A431 epidermoid carcinoma cells using H2O2
A431 cells were plated at 100,000 cells/well in a 96-well plate, and incubated for 24h at 37°C, 5% CO2. After treatment for 10 minutes with increasing concentrations of H2O2, the medium was removed and the cells were lysed with 50 µL of supplemented lysis buffer #3 for 30 minutes at RT under gentle shaking. 16 µL of lysate were transferred into a 384-well low volume white microplate and 4 µL of the HTRF phospho- or total c-Src detection reagents were added. The HTRF signal was recorded after 4 hours of incubation.

Validation on other human cancer cell lines
Culture-treated 96-well plates were seeded with human HepG2 hepatocellular carcinoma cells, human HeLa cervical carcinoma cells and human MCF-7 breast cancer cells. After incubation, cells were lysed with 50 µL of supplemented lysis buffer #3 for 30 minutes at RT under gentle shaking. 16 µL of lysate were transferred into a 384-well low volume white microplate, and 4 µL of the HTRF phospho- or total c-Src detection reagents were added. The HTRF signal was recorded after 4 hours of incubation.

Cell density experiment on mouse NIH/3T3 cells treated with H2O2
Mouse NIH/3T3 embryonic fibroblast cells were plated at different cell densities in a 96-well plate and incubated for 24h at 37°C, 5% CO2. The following day, the cells were treated with increasing concentrations of H2O2 for 10 minutes. The medium was then removed and the cells were lysed with 50 µL of supplemented lysis buffer #3 for 30 minutes at RT under gentle shaking. 16 µL of lysate were transferred into a 384-well low volume white microplate and 4 µL of the HTRF phospho- or total c-Src detection reagents were added. The HTRF signal was recorded after 4 hours of incubation.

Correlation with ELISA on HEK293 cells treated with Bosutinib
HEK293 cells were plated and cultured for 24h. After treatment with increasing concentrations of Bosutinib and H2O2 stimulation for 10 minutes, the medium was removed and the cells were lysed with either ELISA or HTRF kit reagents. The HTRF signal and colorimetric ELISA signal were measured. Similar IC50 values for Bosutinib were obtained using the two methods, demonstrating that HTRF is well correlated to ELISA.

Simplified pathway
c-Src Simpified Pathway
c-Src is a membrane-associated, non-receptor protein tyrosine kinase that is ubiquitously expressed in all cell types. The kinase is inactive when phosphorylated on Tyr530. Dephosphorylation of Tyr530 followed by autophosphorylation on Tyr419 is a hallmark of its full activation. c-Src is mainly activated by RTKs, integrins and ROS, and is a key signaling intermediary regulating numerous pathways such as AKT, MAPKs, STATs and NF?B. It regulates cell proliferation, differentiation, survival, morphology, adhesion and migration. Precise regulation of Src activity is therefore critical for normal cell growth. This derived-proto-oncogene is over-expressed and highly activated in many human cancers (e.g. breast, colon and pancreatic), making it a promising target for cancer therapy.

Resources
Are you looking for resources, click on the resource type to explore further.
This guide provides you an overview of HTRF applications in several therapeutic areas.


How can we help you?
We are here to answer your questions.






























