
Cellometer X2 Fluorescent Cell Viability Counter


Cellometer X2 Fluorescent Cell Viability Counter


Cellometer X2 Fluorescent Cell Viability Counter


The Cellometer™ X2 automated cell counter is optimized for brewing yeast, wine yeast, platelets and other small cells.
Product information
Overview
Our yeast automated cell counter, the Cellometer X2, can be up to five times faster than manual cell counting. The cell count, concentration, diameter, and % viability are automatically calculated and reported within less than 60 seconds.
The Cellometer X2 is optimized for live yeast cell concentration and viability counting. It can also be used for:
- Platelets
- Other small cells
Additional product information
Fluorescent analysis
Fluorescent dyes that stain DNA can be used to identify nucleated cells in a mixed cell population. Acridine orange (AO) is a nuclear staining (nucleic acid binding) dye permeable to both live and dead cells. It stains all nucleated cells to generate green fluorescence when imaged with the Cellometer X2.
Because mature mammalian red blood cells don’t contain nuclei, only mononuclear cells produce a fluorescent signal. Fluorescent-positive nucleated cells are easily counted. There is no need to lyse red blood cells, saving time and eliminating an extra sample preparation step.

With propidium iodide (PI), dead cells are clearly visible in the red fluorescence channel
Use of a fluorescent dye, such as propidium iodide (PI), is highly recommended for accurate viability analysis of cell samples containing debris.
Propidium iodide (PI) is a nuclear staining (nucleic acid binding) dye that enters dead cells with compromised membranes. It stains all dead nucleated cells to generate red fluorescence.
Because the Cellometer X2 can count dead cells in the red fluorescence channel, there is no interference from debris and live cells.
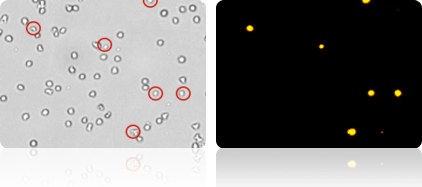
1-Step yeast concentration & viability analysis using Cellometer X2
Brightfield and fluorescent imaging
The Cellometer X2 Image Cytometer performs simple, 1-step determination of yeast concentration and viability. The Cellometer X2 is useful for research laboratories looking to automate fermentation monitoring.
Brightfield images can be viewed to confirm cell morphology. Counted brightfield images show counted cells within cell clumps. Fluorescent images show dead cells for confirmation of viability results. Analysis of both brightfield and fluorescent images from each sample makes 1-step concentration & viability determination possible.
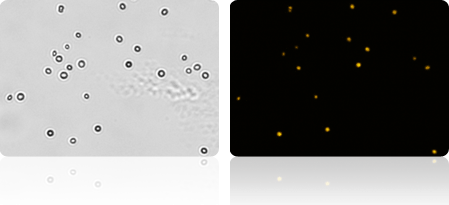
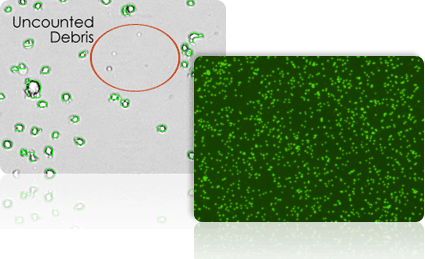
Brightfield and fluorescent images of yeast cells. Cells appearing in both the brightfield and fluorescent image are dead.
Exclusion of debris & size-based cell counting
Because Cellometer recognizes cells based on size, brightness, and morphology, cellular debris is easily and accurately excluded from counting results.
- Cell size parameters can be modified to optimize exclusion of debris from results and enhance the accuracy of counting for a wide range of cell sizes.
- A fluorescent nuclear dye, such as acridine orange, can be used to more easily identify nucleated cells in samples containing debris.
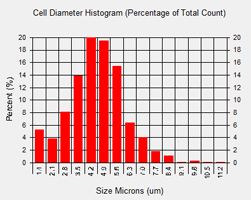
The Cellometer X2 Image Cytometer automatically generates a cell size histogram based on cell diameter.
The minimum and maximum cell diameter settings can be optimized to count specific cells in a sample.
Dynamic range of total cells with Cellometer X2
Concentration dynamic range figure 1 depicts the dynamic range for cell concentration measured by Cellometer X2. This data set was taken on a concentration series of cultured yeasts.
Samples from 2.5 x 105 - 5 x 107 cells/ml can be counted without further dilution.
The %CV at each concentration was below 10%.
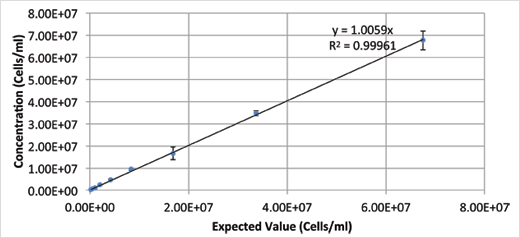
Figure 1: Table of results for cell concentration dynamic range.
Cellometer X2 repeatability and consistency
The results provide evidence of the increased accuracy of the Cellometer X2 instrument in assessing the viability of yeasts using AOPI for cell viability. Yeasts were tested at 24 sample replications. The viability average was calculated and plotted. The results show the reliability and accuracy of the Cellometer X2 in measuring cell concentration and viability of yeast cells.
| Cellometer X2 | Average live cell concentration via fluorescence | Viability |
|---|---|---|
| AVE | 1.32E+07 | 78.1% |
| STDEV | 7.69E+05 | 2.2% |
| %CV | 5.84 | 2.78 |
Table of results for cell concentration and viability using acridine orange (AO) and propidium Iodide (PI).
Cellometer X2 specifications
| Includes |
|
|---|---|
| Available accessories |
|
| Imaging performance |
|
| Instrument specifications |
|
|
PC / Laptop minimum requirements: (If purchasing Cellometer without PC laptop) |
|
| Available fluorescence optics modules |
VC-535-403 Excitation / Emission: 470nm/535nm Example fluorophores:
VC-660-503 Excitation / Emission: 540nm/660nm Example fluorophores:
|
Specifications
| Brand |
Cellometer
|
|---|---|
| Unit Size |
1 each
|
Resources
Are you looking for resources, click on the resource type to explore further.
White paper highlighting cell counting methods and the differences between them.
Cell counting precision is a critical parameter for determining the overall quality of cell counting results. Many factors...
One of the most promising fields in cancer research is immunotherapy – manipulating the existing immune system to specifically...
The hemacytometer is an integral part of all cell-based research, and yet sources of error inherent in its design and utilization...
The importance of cell counting has increased significantly in the last decade due to the major advances in the fields of cell and...
The hemacytometer has been an essential tool for hematologists, medical practitioners, and biologists for over a century. In this...
SDS, COAs, Manuals and more
Are you looking for technical documents related to the product? We have categorized them in dedicated sections below. Explore now.
- Resource TypeManualLanguageEnglishCountry-
- Resource TypeManualLanguageEnglishCountry-


Recently Viewed
How can we help you?
We are here to answer your questions.







































