

HTRF Human Phospho-SLP76 (Ser376) Detection Kit, 10,000 Assay Points


HTRF Human Phospho-SLP76 (Ser376) Detection Kit, 10,000 Assay Points






The phospho-SLP-76 (Ser376) kit enables the cell-based quantitative detection of phosphorylated SLP-76 at Ser376, for monitoring T-lymphocyte activation.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
The phospho-SLP-76 (Ser376) kit enables the cell-based quantitative detection of phosphorylated SLP-76 at Ser376, for monitoring T-lymphocyte activation.



HTRF Human Phospho-SLP76 (Ser376) Detection Kit, 10,000 Assay Points



HTRF Human Phospho-SLP76 (Ser376) Detection Kit, 10,000 Assay Points



Product information
Overview
This HTRF cell-based assay enables rapid, quantitative detection of SLP-76 phosphorylated on Serine 376. Phospho-SLP-76 creates a scaffold on which key signaling complexes are built, and is a marker of T-lymphocyte activation
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
Lysis Buffer 4
Lysis Buffer 5
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Oncology & Inflammation
|
| Unit Size |
10,000 Assay Points
|
Video gallery

HTRF Human Phospho-SLP76 (Ser376) Detection Kit, 10,000 Assay Points

HTRF Human Phospho-SLP76 (Ser376) Detection Kit, 10,000 Assay Points

How it works
Phospho-SLP-76 (Ser376) assay principle
The Phospho-SLP-76 (Ser376) assay measures SLP-76 when phosphorylated at Ser376. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The Phospho-SLP-76 (Ser376) assay uses 2 labeled antibodies: one with a donor fluorophore, the other one with an acceptor. The first antibody is selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the proteins phosphorylation state under a no-wash assay format.

Phospho-SLP-76 (Ser376) 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding Phospho-SLP-76 (Ser376) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-SLP-76 (Ser376) 1-plate assay protocol
Detection of Phosphorylated SLP-76 (Ser376) with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
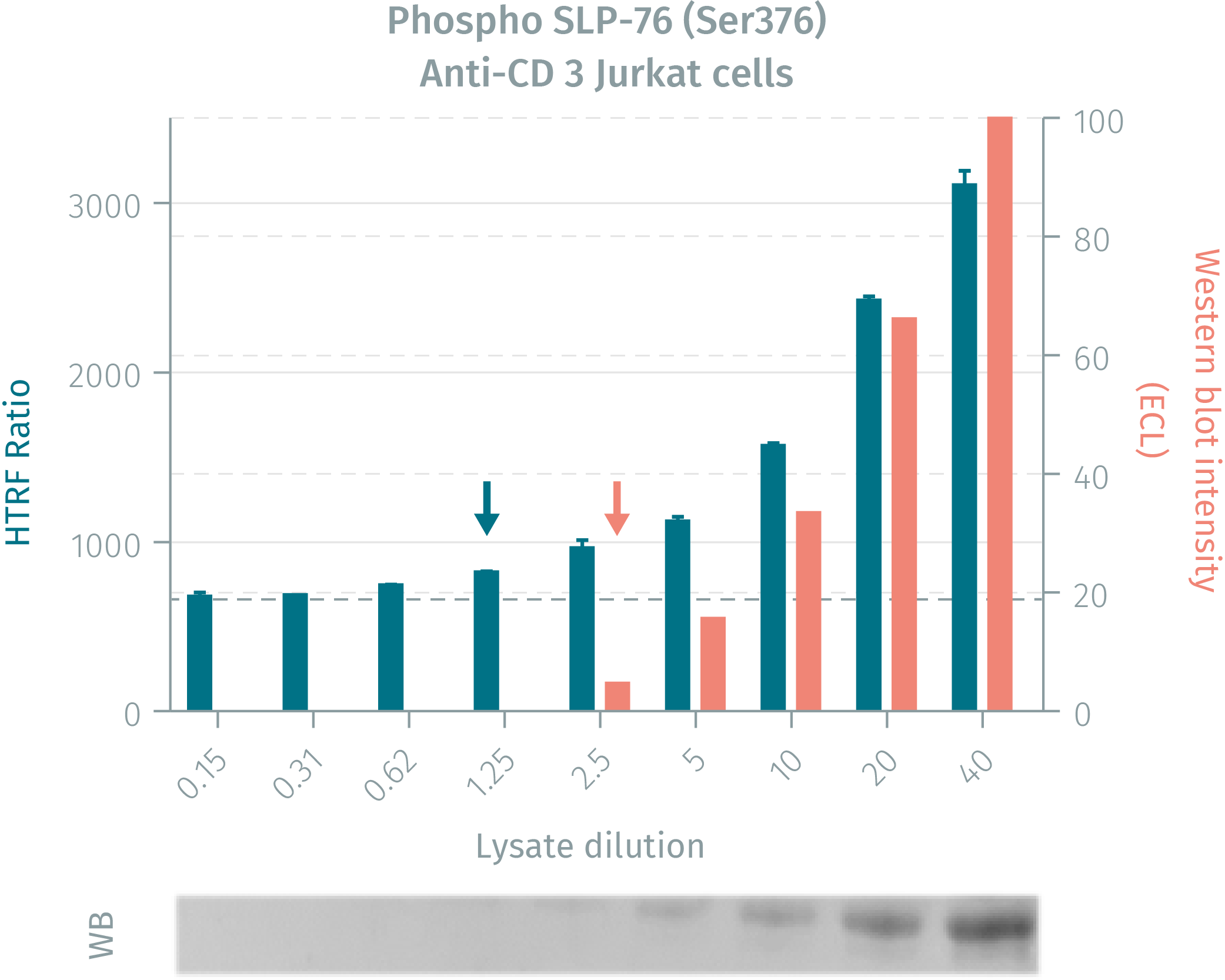
HTRF assay compared to western blot using phospho SLP-76 (Ser376) cellular assays on human jurkat cells
Jurkat cells were grown in a T175cm2 flask for 2 days in RPMI culture medium supplemented by 10% FBS, at 37°C in 5% CO2 atmosphere. 4.5 mL of cell suspension (13 x 106 cells / ml) were stimulated with anti-CD3 antibody (20µg/mL final concentration during 2.5min). After stimulation, the cells were lysed at RT for 30 min by adding 2.25 mL of 4X supplemented lysis buffer. Neat lysate was then serially diluted in the same supplemented lysis buffer. 16 µL of each dilution were analyzed in parallel by HTRF or by Western Blot.
Cell density optimisation for phospho SLP-76 (Ser376)
This cell density optimization was carried out using the two plate protocol. Jurkat cells were plated in a 96-well culture plate at 200,000 Kcells or 400Kcells in 25µL/well, in complete RPMI culture medium with 10% FBS. Cell stimulation was done by anti-CD3 Ab at various concentrations for 30 min. Cell lysis was performed using 10 µL of 4X supplemented lysis buffer. 16 µL of each cell lysate were analyzed by HTRF Phospho SLP-76(Ser376) assays

Kinetics of cell stimulation
This assay was carried out with a two plate protocol. 400,000 Jurkat cells per well were plated in a 96-well culture plate at 25µL/well in complete RPMI culture medium with 10% FBS. Cell stimulation was performed using anti CD3 Ab at the final concentration of 20µg/mL for different stimulation times (min). After stimulation, cells were lysed with 10 µL of 4X supplemented lysis buffer. 16 µL of each cell lysate for the different conditions were analyzed by HTRF Phospho SLP-76(Ser376) and Total SLP-76.

Monitoring of the immune checkpoint inhibitor PD-L1 activity
As Phospho SLP-76 (Tyr376) is a readout of T cell activation, it was used to monitor the inhibitory effect of PD-L1 recombinant protein on T cell activation. This assay was performed using the two-plate assay protocol. 400 000 Jurkat cells were plated in 25 µL of complete RPMI culture medium with 10% FBS. Cells were pre-incubated 24 hours with different concentrations of PD-L1 recombinant protein. Cells were then stimulated for 2.5 min with 20 µg / mL anti-CD3 Ab (final concentration) and lysed directly with10 µL of 4X supplemented lysis buffer, then incubated 30 min at RT. 16µL of each sample were analyzed using Phospho SLP-76 (Ser376) and Total SLP-76 assays. Phospho SLP-76 results were normalized with the Total SLP-76 amount and plotted on a graph.
A slight but significant inhibitory effect of PD-L1 recombinant protein was recorded with the Phospho SLP-76 (Ser376) assay at the final concentration of 40 µg/mL

Simplified pathway
Function and regulation of SLP-76
TCR engagement promotes the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) on the cytosolic side of the TCR/CD3 complex by lymphocyte protein tyrosine kinase (Lck). Zap-70 is recruited to the TCR/CD3 complex, where it becomes phosphorylated and activated. ZAP-70 phosphorylates SLP-76. Activated SLP-76 translocates to the plasma membrane and promotes a multi-protein complex, which participates in the activation, survival, and proliferation of T-Lymphocytes

Resources
Are you looking for resources, click on the resource type to explore further.
Advance your autoimmune disease research and benefit from Revvity broad offering of reagent technologies


How can we help you?
We are here to answer your questions.






























