

HTRF Human & Mouse Phospho-Histone H3 (Ser10) Detection Kit, 500 Assay Points


HTRF Human & Mouse Phospho-Histone H3 (Ser10) Detection Kit, 500 Assay Points






Histone H3 P-s10 Kit - 50,000 Tests
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
Histone H3 P-s10 Kit - 50,000 Tests



HTRF Human & Mouse Phospho-Histone H3 (Ser10) Detection Kit, 500 Assay Points



HTRF Human & Mouse Phospho-Histone H3 (Ser10) Detection Kit, 500 Assay Points



Product information
Overview
The Phospho-Histone H3 (Ser10) assay enables the detection of Ser10 phosphorylation in the tails of Histone H3, a key regulator of gene expression and mitosis. Phosphorylation at Ser10 is tightly correlated with chromosome condensation during both mitosis and meiosis.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Neuroscience
|
| Unit Size |
500 Assay Points
|
Video gallery

HTRF Human & Mouse Phospho-Histone H3 (Ser10) Detection Kit, 500 Assay Points

HTRF Human & Mouse Phospho-Histone H3 (Ser10) Detection Kit, 500 Assay Points

How it works
Phospho-Histone H3 (Ser10) assay principle
The phospho-Histone H3 (Ser10) assay measures Histone H3 when phosphorylated at Ser10. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The phospho-Histone H3 (Ser10) assay uses 2 labeled antibodies: one with a donor fluorophore, the other one with an acceptor. The first antibody is selected for its specific binding to the phosphorylated motif on the protein, the second for its ability to recognize the protein independent of its phosphorylation state. Protein phosphorylation enables an immune-complex formation involving both labeled antibodies and which brings the donor fluorophore into close proximity to the acceptor, thereby generating a FRET signal. Its intensity is directly proportional to the concentration of phosphorylated protein present in the sample, and provides a means of assessing the proteins phosphorylation state under a no-wash assay format.

Phospho-Histone H3 (Ser10) 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding Phospho-Histone H3 (Ser10) HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Phospho-Histone H3 (Ser10) 1-plate assay protocol
Detection of Phosphorylated Histone H3 (Ser10) with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
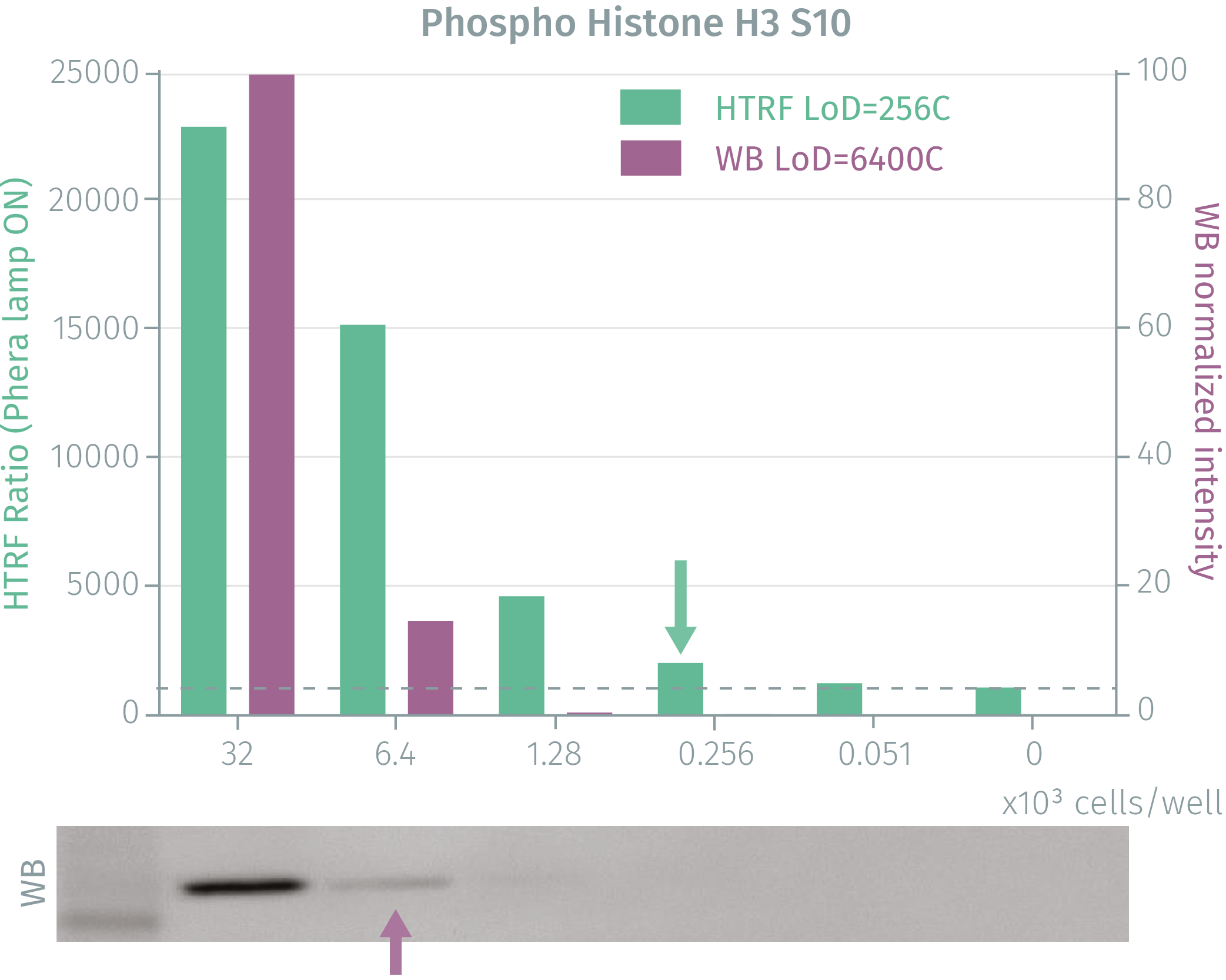
HTRF phospho-Histone H3 (Ser10) assay compared to Western Blot
Exponentially growing Hela cells were cultured to 80% confluency and treated with Nocodazole for 16 hours. After medium removal and lysis, soluble supernatants were collected via centrifugation. Equal amounts of lysates were used for a side by side comparison of WB and HTRF. The HTRF assay is 25-fold more sensitive than the Western Blot. Using HTRF phospho-Histone H3 (Ser10), only 256 cells are sufficient for minimal signal detection, while 6,400 cells are needed for a Western Blot signal. The HTRF assay is 25-fold more sensitive than the Western Blot.
Phospho-Histone H3 (Ser10) as a mitotic biomarker for cell proliferation
Hela and A431 cells were seeded in a 96-well plate and treated for 16 hours with Nocodazole, an antimitotic agent that depolymerizes microtubules and blocks the cells in mitosis, when Histone H3 Ser10 phosphorylation is maximal. After cell culture media removal, 50 µl, 100 µl or 200 µl of lysis buffer were added for a 30-minute incubation. 16 µl of each lysate were transferred to a 384sv white plate for HTRF analysis. Increasing the lysis volume up to 200µl improved the linearity of the HTRF detection signal.


Phospho-Histone H3 (Ser10) as read-out for Aurora Kinase B inhibition



Simplified pathway
Phospho-Histone H3-Ser10 in the cell cycle
Overall histone phosphorylation fluctuates very markedly during the cell cycle and is characterized by peaks in the phosphorylation of Histone H3 on Serine10 in mitosis. Aurora kinase B is directly responsible for the mitotic Histone H3 Ser10 phosphorylation. Aurora kinase A, in complex with BORA, is responsible for the initial activation of PLK1 in G2 phase, leading to the activation of cyclin-dependent kinase 1 mitotic entry at the G2/M transition checkpoint. Aurora kinase A is frequently amplified in human cancers, and Aurora kinase A overexpression in human tumours correlates with a poor prognosis and genomic instability. Moreover, high expression levels of Aurora kinase B are associated with a poor prognosis in glioblastoma, ovarian carcinoma and hepatocellular carcinoma.

Resources
Are you looking for resources, click on the resource type to explore further.
This guide provides you an overview of HTRF applications in several therapeutic areas.


How can we help you?
We are here to answer your questions.






























