Overview
We currently offer two LANCE™ kits for cAMP detection: the LANCE™ Ultra cAMP kit, and the LANCE™ cAMP kit. The LANCE Ultra cAMP kit is our newer, two-component assay. The LANCE Ultra cAMP kit uses a ULight-dye labeled monoclonal anti-cAMP antibody and has improved sensitivity, signal stability, pharmacological stability, and signal-to-noise compared to the LANCE cAMP kit. This can be helpful if you are working with difficult targets, Gi-coupled receptors, endogenous receptors, low cell numbers, or if you are screening. This page contains information about the LANCE Ultra cAMP kit. You can also view information for our original LANCE cAMP kit.
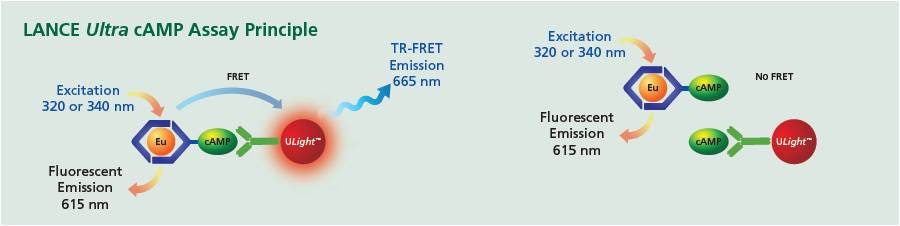
The LANCE™ Ultra cAMP assay is a homogeneous time-resolved fluorescence energy transfer (TR-FRET) immunoassay designed to measure cAMP produced upon modulation of adenylyl cyclase activity by GPCRs. It is a functional assay for Gs-coupled and Gi-coupled GPCR studies. The assay is based on the competition between a Europium-labeled cAMP tracer and sample cAMP for binding sites on cAMP-specific antibodies labeled with ULight dye.
When the ULight anti-cAMP antibody is bound to the Eu-cAMP tracer, excitation at 340 nm excites the Europium. The energy is transferred to the ULight-labeled antibody. The fluorescence measured at 665 nm will decrease in the presence of cAMP from test samples, and resulting signals will be inversely proportional to the cAMP concentration of a sample (this is a competition assay) (Figure 1). The assay is intended for the detection of cAMP produced by cells or cell membrane preparations stimulated with GPCR agonists.

Figure 1. LANCE Ultra cAMP competition assay principle. Left panel: In the absence of cellular cAMP, the Eu-cAMP probe binds to the ULight-anti-cAMP antibody to generate maximum signal. Right panel: With increasing concentrations of free cAMP (cellular cAMP), the free cAMP can bind the antibody instead, resulting in a decrease in signal.
Gs-coupled and Gi-coupled receptors
For Gs-coupled receptors, agonist stimulation of cells or membrane preparations results in an increase in cAMP levels, and a decrease in assay signal (this is a competition assay). Addition of an antagonist will reverse the response, resulting in an increase in signal.
For Gi-coupled receptors, cells are simultaneously stimulated by the adenylyl cyclase activator, forskolin, and by the agonist. An agonist will inhibit the forskolin-induced cAMP production, resulting in an increase in signal compared to forskolin alone (competition assay). Antagonists will block the effect of the agonist, resulting in a decrease in signal toward forskolin-induced levels.
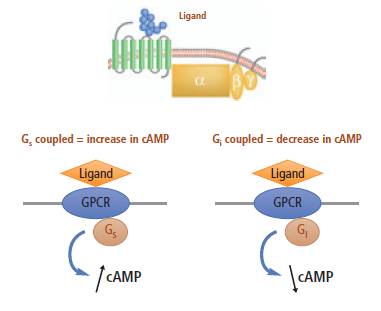
Gi and Gs-coupled receptors.
What do I need to run this assay?
Required reagents available from Revvity:
- LANCE Ultra cAMP kit (catalog number TRF0262, TRF0263, or TRF0264)
- Microplates (we recommend white OptiPlate™ microplates.)
- OptiPlate-96 (96-well) catalog number 6005290
- OptiPlate-384 (384-well) catalog number 6007290
- OptiPlate-1536 (1536-well) catalog number 6004290
- Seal™-A adhesive plate seal (catalog number 6005250)
- A cell line or membrane preparation expressing your GPCR of interest (View Revvity cell lines and membranes; you can also use your own cells or cell membrane preparations.)
Required reagents available from other suppliers:
(See suggested sources for these reagents.)
- Versene™ for cell detachment
- Appropriate media
- Other components to make stimulation buffer (1X HBSS, 5 mM HEPES)
- IBMX
- Forskolin (if working with Gi-coupled receptors)
Instrumentation/Equipment:
- A TRF-capable plate reader (We recommend the EnVision™ or VICTOR™ or ViewLux™ Multilabel Plate Reader.)
Protocol-in-brief
The general protocol for the LANCE Ultra cAMP assay is summarized in Figure 2. Please refer to the manual for a more detailed protocol.
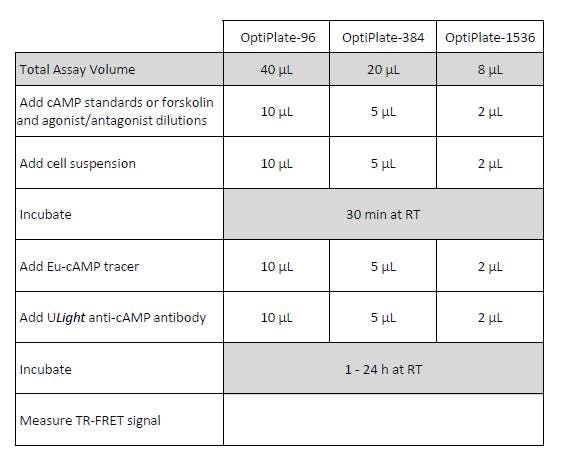
Figure 2. LANCE Ultra cAMP protocol.
Assay optimizations
View our LANCE Ultra cAMP assay development guide. This guide contains detailed information on how to optimize a LANCE Ultra cAMP assay. The assay development guide also includes information on cell handling, suggested platemaps for each optimization step, detailed protocols for running agonist and antagonist assays on Gi- or Gs-coupled receptors, and information on data interpretation and protocol alterations.
If you are switching from a different kit, we recommend that you re-optimize the number of cells used in the assay, as well as other parameters such as forskolin concentration (if applicable). The LANCE Ultra cAMP kit is more sensitive than other TR-FRET cAMP assays, and fewer cells will be needed.
These parameters should be optimized for each GPCR cell line:
- Cell number
- Forskolin concentration (if working with Gi-coupled receptors)
- Agonist concentration
- Stimulation time
Application notes, posters, and guides
- LANCE Ultra cAMP Assay Development Guide - contains detailed information on how to optimize a LANCE Ultra cAMP assay, information on cell handling, suggested platemaps for each optimization step, detailed protocols for running agonist and antagonist assays on Gi- or Gs-coupled receptors, and information on data interpretation and protocol alterations.
- LANCE Ultra cAMP manual - contains assay protocol and instrument settings
- Application note - miniaturization of a LANCE Ultra cAMP assay for the 5-HT1A (Gi-coupled) receptor. The LANCE Ultra cAMP assay was compared against a cAMP assay from another company in both 384-well and 1536-well formats.
- Poster - cAMP assays in primary cells
- Poster - phosphodiesterase assay using LANCE Ultra cAMP
Citations
View a brief list of LANCE cAMP citations.
Tips and FAQs
- If you are switching from a different cAMP kit, we recommend you re-optimize the number of cells used in your assay. The LANCE Ultra cAMP kit is sensitive in comparison to other kits, and usually works well using 500 - 2000 cells in 384-well format. We also would recommend you re-verify the forskolin concentration (if applicable) to see better results.
- Note that you cannot use 10X LANCE Detection buffer (#CR97-100) in a LANCE Ultra cAMP assay. The Detection buffer included in the LANCE cAMP kits is a different formulation, that contains detergent for cell lysis.
- Reagents supplied with this kit should be used together. To maintain assay performance between lots, do not mix reagents from kits with different lot numbers.
- Briefly microfuge vials of reagents to improve recovery of the contents.
- Mix gently ULight-labeled cAMP solutions. Do not vortex.
- The use of OptiPlate white opaque microplates is strongly recommended. The use of black plates will result in a reduced signal but acceptable S/B ratios.
- It is critical to remove the Seal-A film from the plate prior to reading.
- A special grade of BSA (7.5% Stabilizer solution) specifically designed for LANCE applications is provided with the kit. Do not use another source of BSA as this might result in unreliable data. This 7.5% Stabilizer solution is available as a standalone product (catalog number CR84-100).
Q. Do I have to lyse my cells?
A. The Detection buffer included in the kit contains a detergent (Triton X-100) that will lyse most cells.
Q. Do I need to use IBMX?
A. If you are using cells, yes. IBMX is a phosphodiesterase inhibitor and prevents the degradation of cAMP.
Q. Why am I using forskolin?
A. If you are studying a Gi-coupled receptor, you will be looking for a decrease in cAMP levels upon stimulation of your receptor. To raise the baseline levels of cAMP for unstimulated cells, you will treat all samples with forskolin. Forskolin stimulates adenylyl cyclase, which will trigger cAMP production.
Q. How do I pick a forskolin concentration?
A. Refer to pages 9-11 of the LANCE Ultra cAMP Assay Development Guide.
Q. What is the minimum receptor expression level required?
A. There is no minimum receptor expression level required. Some endogenous receptors were shown to induce the release of high amounts of cAMP. Tight coupling between receptors, G-proteins (Gi or Gs) and adenylate cyclase are the most critical determinants for obtaining robust cAMP assays. The LANCE Ultra cAMP assay is extremely sensitive and will allow measuring receptor stimulation in cells producing low levels of cAMP.
Q. Should I use transient or stable transfected cell lines?
A. Both transient and stable cell lines have been shown to produce good responses upon adenylate cyclase activation.
Q. Can I use frozen (irradiated, non-propagatable) cell lines?
A. Frozen cell lines, including cAMPZen™ from Revvity, have been shown to produce excellent responses upon adenylate cyclase activation.
Q. Can I run the assay while my cells are adhered to the plate?
A. Yes, cells can be treated while adherent if you prefer. Here is a protocol used by our R&D scientists working with adherent cells:
- Cells were plated the day before. CHO cells expressing the h5HT1a receptor were cultured in MEM media supplemented with 10% heat inactivated fetal bovine serum and 2 mM Glutamine. Twenty-four hours prior to the experiment (~90% confluency in the T-75 cm2), the cells were detached using the HYQTase method. Cells were counted and plated in a CulturPlate-384 plate (Revvity Cat. No. 6007680) at 1,000 and 2,000 cells per well with 50 μL of culture media. Because CulturPlates are opaque, cells were plated in parallel in clear-bottom plates (ViewPlate-384 TC plates, Cat. No. 6007460) to assess cell density and monitor growth.
- The media for the cells grown in CulturPlate-384 plates was removed by inverting the plate, and cells were washed manually (with a multi-channel pipettor) twice with 60 μL of HBSS to eliminate residual media.
- Then the assay was run as described in the user manual, stimulating cells for 30 min in stimulation buffer containing forskolin, agonist and antagonist.
Q. Can I run LANCE Ultra cAMP assays in culture media, rather than stimulation buffer?
A. Yes, though keep in mind the TR-FRET signal might be somewhat quenched by components in your culture media. See the figure below for an example.
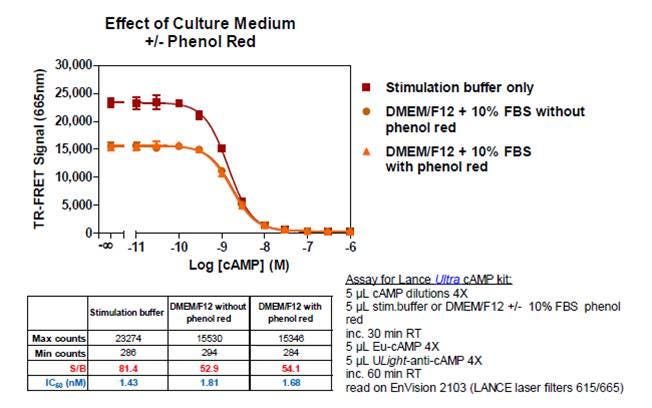
Q. Can I run LANCE Ultra cAMP assays in culture media, rather than stimulation buffer?
A. Yes, though keep in mind the TR-FRET signal might be somewhat quenched by components in your culture media. See the figure for an example. Figure 3. LANCE Ultra cAMP standard curve run in stimulation buffer vs. culture media.
Q. How many cells should I use in my assay?
A. Cell number will influence the cAMP levels before (basal) and after adenylate cyclase activation. Performing a cell titration allows you to optimize the signal window by maximizing the difference between the basal and stimulated counts.
Q. Is it necessary to perform a time course for cell stimulation?
A. The stimulation time is critical for reaching optimal detection of cAMP. When determining the optimal cell density for the assay, we recommend stimulating the cells for 30 minutes as a first trial. Once the optimal cell density has been determined, perform a time course experiment with stimulation times ranging from 15 minutes to 120 minutes. The time of stimulation may vary depending on the cell line, receptor and agonist being studied.
Q. Can attached cells be used?
A. Attached cells or detached cells can be used. However, we recommend rinsing attached cells with HBSS 1X to remove cell culture medium prior to using them in the LANCE Ultra cAMP assay.
Q. Can membrane preparations be used instead of whole cells?
A. Membranes expressing Gs-coupled receptors have been shown to produce good results when the stimulation buffer is supplemented with appropriate ions. 1-5 μg membranes are typically used in such assays. We recommend titrating membranes and all supplemented components (e.g. MgCl2, GTP, GDP and ATP) to optimize the performance of membrane-based assays.
Q. Can I decrease the number of additions (pipetting steps) in the assay?
A. Yes, if you wish to decrease the number of pipetting steps, you can mix the anti-cAMP antibody with your cells in stimulation buffer and add this mix to the plate in one pipetting step at the beginning of the assay. Do not use Detection buffer to dilute the anti-cAMP antibody if you choose to run the assay this way - the Detection buffer will lyse your cells. Make sure you adjust the concentration of your reagents so that you have the same final concentrations in the assay.
Q. Does IBMX interfere with the assay?
A. IBMX is a well-known phosphodiesterase inhibitor. We recommend using IBMX at a concentration of 0.5 mM in stimulation buffer. At this concentration, IBMX does not reduce the signal in cAMP standard curves. The IBMX concentration may need further optimization in cell-based assays when working with different cell lines.
Q. Does DMSO interfere in the assay?
A. DMSO at concentrations up to 2% during cell stimulation does not affect assay performance. However, the DMSO concentration should be titrated for each cell line used.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























