
Overview
Protein phosphorylation and dephosphorylation events are involved in the regulation of many aspects of the cell cycle, including proliferation, differentiation, secretion and apoptosis. Aberrant protein phosphorylation is a cause or consequence of many human diseases. As a result, kinases and phosphatases have become critical targets for drug discovery and this has prompted the development of many assay technologies suitable for high-throughput screening (HTS). LANCE™ Ultra time-resolved fluorescence energy transfer (TR-FRET) reagents were developed primarily for detecting Ser/Thr and Tyr phosphorylation events. An extensive series of Europium chelate (Eu)-labeled anti-phospho antibodies were developed with their specific unphosphorylated ULight ™-labeled peptide counterparts. In the classical LANCE Ultra homogeneous assay set-up, peptide phosphorylation events cause an increase of the TR-FRET signal while kinase inhibitors lead to signal decrease.
In LANCE Ultra assays, Eu-labeled anti-phospho-target antibodies bind to phosphorylated ULight -labeled substrates, which brings Eu donor and ULight acceptor dye molecules into close proximity. Following excitation of the assay reaction at 320 or 340 nm, the energy from the Eu donor is transferred to the ULight acceptor dye which, in turn, emits light at 665 nm. The intensity of the emission output is proportional to the level of ULight -substrate phosphorylation. In LANCE Ultra phosphatase assays, peptide phosphorylation decreases over time, resulting in a reduced interaction between the Eu-labeled antibodies and ULight -labeled peptides. Phosphatase activity thus leads to TR-FRET signal decrease, while phosphatase inhibitors prevent this decrease in a concentration dependent manner.

Figure 1: LANCE Ultra phosphatase assay principle: peptide phosphorylation decreases over time, resulting in a reduced interaction between the Eu-labeled antibodies and ULight -labeled peptides. Phosphatase activity thus leads to TR-FRET signal decrease, while phosphatase inhibitors prevent this decrease in a concentration-dependent manner.
What do I need to run this assay?
- ULight-phospho-peptide substrate, available through OnPoint custom services
- Eu-anti-P-Tau (pThr231), available through OnPoint custom services
- Eu-anti-P-oIIa (pThr1342) TRF0218-D
- Eu-anti-P-4E-BP1 (pThr46) TRF0216-D
- Eu-anti-PT66 (anti-P-tyrosine) AD0068
- Eu-anti-phospho substrate antibody
- 10X LANCE Detection Buffer CR97-100
- Microplate (We recommend white OptiPlates or ProxiPlates™)
- Seal-A™ adhesive seal for microplates 6050185
-
A TR-FRET-capable plate reader (We recommend the Revvity EnVision™ or VICTOR™ Plate Reader.)
- All phosphatases (human recombinant) were from Millipore
- Concentrations of peptides are calculated for 10 µL reactions, while antibody concentrations are for 20 µL total assay volumes
- Assay Buffer: 50 mM HEPES pH 7.5, 1 mM EGTA, 10 mM MgCl2, 3 mM MnCl2, 2 mM DTT, and 0.01% Tween-20
Protocol-in-brief
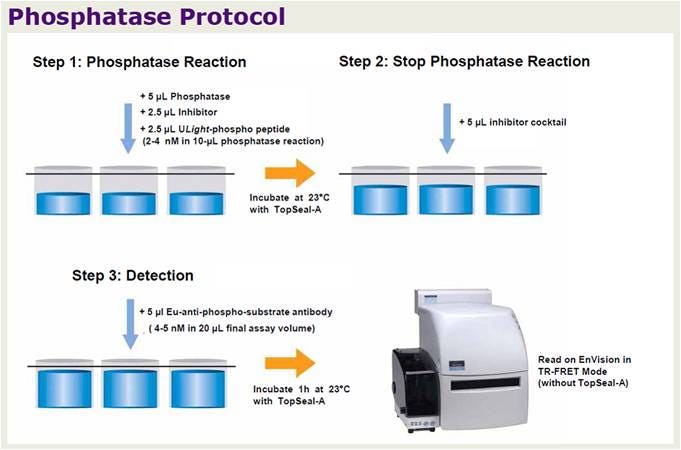
LANCE TR-FRET phosphatase assay workflow.
Assay optimizations
The data below are shown as proof-of-concept and are intended to provide guidance on how you can optimize any LANCE phosphatase assay. Please note that the anti-phospho-Tau (Thr231) antibody used in these experiments is no longer available. However, Revvity offers a variety of LANCE Europium-labeled anti-phospho antibodies as part of our product catalog that can be used to develop a similar phosphatase assay using a matching pre-phosphorylated ULight peptide substrate. Additionally, we offer LANCE Europium labeling reagents if you would like to Europium-label your own antibody. We also offer custom labeling services if you would like for us to Europium-label an antibody for you.
Optimization of antibody/phospho-peptide ratio
In phosphatase signal-decrease assays, initial enzymatic conditions are critical for the development of a sensitive assay. If the amount of labeled substrate is too high relative to the Eu-antibody concentration, small changes in phosphorylation may remain undetected. In phosphatase signal-decrease assays, ULight -phospho-peptide and Eu-anti-phospho-substrate antibody concentrations must thus be carefully titrated in order to allow detection of low levels of dephosphorylation.
Optimal concentration of TAU (pThr231) phospho-peptide was determined above using 5 nM Eu-anti-phospho-TAU antibody. Different amounts of total TAU peptides, including either 100% or 50% of phosphopeptide, were measured to evaluate the extent of signal decrease in samples containing 50% phospho-peptide (Figure 2). We wanted to determine which peptide concentrations would generate ~50% net signal reduction with 50% of phospho-peptide while yielding a high TR-FRET signal. Total concentrations of peptides ranging from 2 to 5 nM appeared suitable. As shown on Figure 3, a standard curve of TAU phospho-peptide was made by mixing 4 nM total of phospho and non-phospho TAU in different proportions with 5 nM of Eu-anti-phospho-TAU antibody. Excellent linearity was observed (r 2 value of 0.9950), indicating that these peptide and antibody concentrations are ideal for monitoring peptide dephosphorylation in a signal decrease assay.
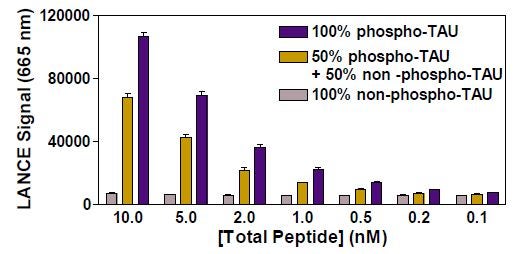
Figure 2: Detection of ULight-Phospho-TAU mixed with ULight -Non-Phospho TAU Peptides and 5 nM Anti-Phospho-TAU Antibody
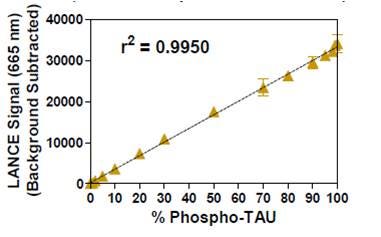
Figure 3. Detection of ULight -Phospho-TAU mixed with ULight -Non-Phospho TAU Peptides and 5 nM Anti-Phospho-TAU Antibody
Serine/Threonine phosphatase assays
PP1A and PP2A Ser/Thr assays
PP1A and PP2A assays were performed with ULight -TAU (pThr231), OIIa (pThr1342) and 4E-BP1 (pThr46) peptides. Various enzyme concentrations were tested (Figure 4). Selected PP1A and PP2A concentrations are highlighted in the figure legend. The pattern of signal decrease was similar to the ones observed in assays using 32 P radio-labeled peptides, but the LANCE Ultra assays used much less enzyme (not shown). S/B ratios for the PP1A and PP2A phosphatase assays were of ~3 for ULight -TAU and OIIa and ~8 for ULight-4E-BP1.
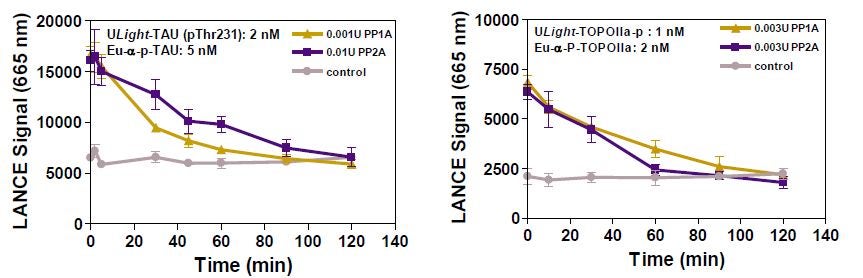
Figure 4: Time-Course of PP1A and PP2A Activity with ULight -4E-BP1 (pThr46)
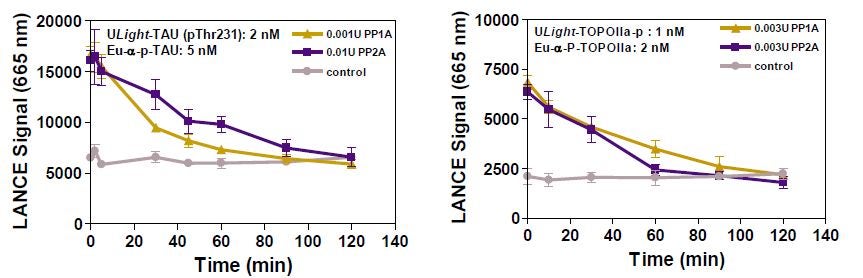
Figure 5: Time course of PP1A and PP2A activity with ULight -TAU (pThr231) is shown in the left panel and the time course of PP1A and PP2A activity with ULight -OIIa (pThr1342) is found in the right panel.
PP2A assay: Okadaic acid dose-response curves
Dose-response curves were performed with PP2A using ULight -OIIa (pThr1342) and 4E-BP1 (pThr46) as substrates. For OIIa, 0.004U of PP2A was used for 90 min. For 4E-BP1, 0.001U of PP2A was used for 60 minutes. Assays gave reproducible IC 50 values (Figure 6: left vs. right panels). Ab and peptide concentrations were slightly modified for the OIIa assay: shown left 2 nM Ab and 1 nM peptide; shown right 3 nM Ab and 2 nM peptide. These concentration changes yielded higher signal but similar S/B ratios and IC 50 values.
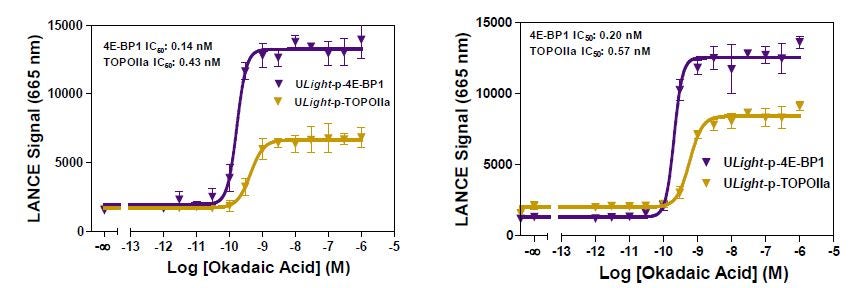
Figure 6: Okadaic acid dose-response curves were performed with PP2A using ULight -OIIa (pThr1342) and 4E-BP1 (pThr46) as substrates.
PP2A assay: Z’ Study
PP2A assay robustness was evaluated in a Z' factor analysis with the ULight-4E-BP1 and ULight-OIIa phosphorylated peptides (Figure 7). Okadaic acid was used at a concentration inhibiting 100% of the enzyme activity (100 nM). Z’ factor was calculated using the PP2A (gold circles) and PP2A + Okadaic Acid (purple circles) data. Controls were the phospho- or non-phospho-peptides alone (open symbols). The two assays gave Z’ factors above 0.7.
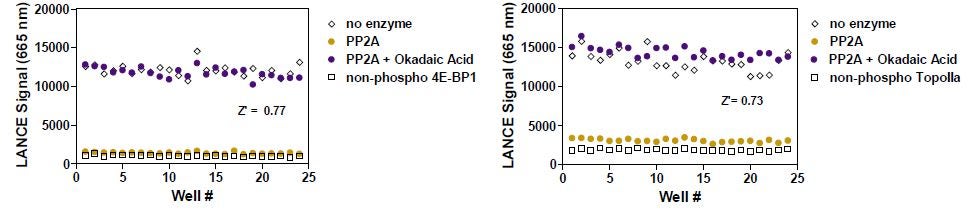
Figure 7: Z' study of the PP2A assay with two substrates. PP2A with ULight -4E-BP1 (pThr46) is found in the left panel and PP2A with ULight -OIIa (pThr1342) is shown in the right panel.
Tyrosine Phosphatase Assays
A similar assay design was applied to measure TCPTP tyrosine phosphatase activity. JAK1 is reported in the literature to be a natural substrate for TCPTP. Figure 8 shows that TCPTP can indeed dephosphorylate the ULight -JAK1 (pTyr1023) peptide. For this assay, we used the generic Eu-labeled-PT66 generic anti-phospho-tyrosine antibody. Although signal decrease is observed, assay background is relatively high, indicating that further assay optimization might be needed. The amount of enzyme sufficient for this experiment is below one picogram per well.
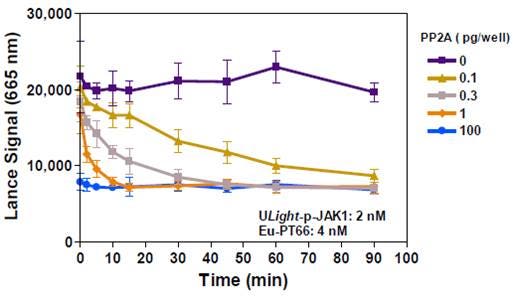
Figure 8: Time-Course of TCPTP Activity with ULight -JAK1 (pTyr1023)
Conclusion
Using the LANCE Ultra technology, we have developed three Ser/Thr and one Tyr Phosphatase assays involving the phosphorylated versions of four LANCE Ultra ULight -peptides derived from TAU (residues surrounding Thr231), 4E-BP1 (residues surrounding Thr46), OIIa (residues surrounding Thr1342) and JAK1 (residues surrounding Tyr1023) as peptide substrates. The optimized PP2A/ULight -OIIa (pThr1342) and PP2A/ULight -4E-BP1 (pThr46) assays were reproducible and robust, as confirmed by Z’-factors above 0.7 in 384-well format.
The IC50 values obtained for the inhibition of PP2A by okadaic acid were of the same order as that reported in the literature (IC50 value = 0.1nM). Average values obtained for P-4E-BP1 and P-OIIa were of ~0.2 and 0.5 nM, respectively. These data suggest that the use of a variety of phosphorylated substrates might allow for pathway selective inhibitor screening. Preliminary work with the TCPTP/ULight -JAK1 (pTyr1023) assay shows that tyrosine phosphatase LANCE Ultra assays can be performed using a generic Eu-labeled anti-phospho-Tyr antibody, such as PT66.
Overall, our results demonstrate the LANCE Ultra technology is well suited for the development of robust and sensitive signal decrease phosphatase assays and for characterizing the effect of their inhibitors.
Posters and other resources
Poster for LANCE Ultra phosphatase assays - describes Ser/Thr phosphatase assays using PP1A and PP2A and Tyr phosphatase assays using TCPTP
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.
Custom labeling and custom assay development services at Revvity
Revvity offers custom labeling services as well as custom assay development. If you are interested in custom phosphorylated ULight substrates, custom LANCE Europium-labeled antibodies, custom barcoded microplates, or in custom assay development, please contact our custom teams:
Custom Labeling and Conjugation Services
Custom Assay Development Services




























