Overview
We currently offer labeling reagents for you to label your biomolecule with a LANCE™ Europium chelate. We also offer custom labeling services if you would prefer that we perform the Europium labeling, or if you need a reagent to be labeled with ULight™ dye.
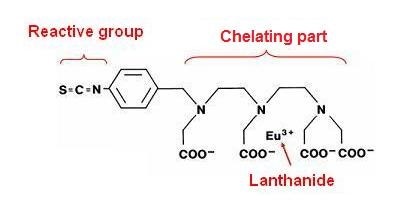
Chelate structure
LANCE chelates can be broken down into three parts:
1. The reactive group. The reactive group dictates what chemical group on your sample will be conjugated/labeled.
- ITC (isothiocyanate) reacts with free amines, including the N-terminus of a protein or peptide, and lysine residues.
- Iodoacetamide reacts with free sulfhydryl groups (cysteines).
- Amino reacts with free carboxyl groups.
- DTA (dichlorotriazine) reacts with free amino groups, sulfhydryl groups, and hydroxyl groups. Because of its high reactivity, we usually do not recommend DTA as it may “inactivate” the reagent you are labeling.
2. The chelating part. The chelating part dictates the fluorescent properties and stability of the LANCE chelate.
- W1024 and W1284: easy labeling, best signal-to-background in FRET assays, best suited for assay conditions where concentration of EDTA is less than 20 mM and pH is greater than 7.
- W8044: more stable, can be used in assays with concentration of EDTA up to 250 mM (under pH neutral conditions), or when the pH of your assay is low (down to pH 4).
3. The Europium lanthanide. You will excite LANCE Europium at 320 or 340 nm.

Chemical structure of a DELFIA™ Eu-labeling reagent, N1-(p-isothiocyanatobenzyl)-diethylene-triamine-N 1,N 2,N 3,N 3-tetracetic acid chelated with Eu3+. The DELFIA chelate is depicted as an example; please note that it is not possible to use DELFIA chelates in LANCE TR-FRET assays.
LANCE Europium chelate table
| Catalog number | Product | Size | Sample | Reactive with | Assay pH stability | Assay tolerance for EDTA / chelators | MW of Europium chelate | Residual molecular weight |
|---|---|---|---|---|---|---|---|---|
| AD0096 | Eu-W1024-ITC chelate | 100 µg | Proteins/peptides | Free amino groups | pH > 7 | Moderate | 697 Da | 697 Da |
| AD0013 | Eu-W1024-ITC chelate | 1 mg | Proteins/peptides | Free amino groups | pH > 7 | Moderate | 697 Da | 697 Da |
* If you do not see your chelate-of-interest, please inquire with our custom teams (contact information is below). We do offer other, more-rare, LANCE Europium chelates upon request.
Protocols
Detailed protocols are provided in each product manual.
Application notes
Application note for LANCE Europium chelate stability.
FAQs
Q. Can I use dialysis to purify away free Europium?
A. Dialysis is not sufficient for removing free Europium. This is not recommended.
Q: Can I use spin columns to purify my labeled reagent?
A: We have tried to use spin columns in the past and the result was that unreacted chelate wasn't removed efficiently enough.
Q. Can I use desalting columns to purify my labeled reagent?
A. Desalting columns don't work with LANCE chelates.
Q. Which chelate should I use?
A. We usually recommend our Eu-W1024 chelate. This chelate gives the best signal-to-background (S:B) in a LANCE TR-FRET assay. The LANCE Eu-W1024-ITC labeling reagent will react with lysines and free N-termini. If you require a particularly stable chelate because your assay will be run at lower pH or in the presence of high amounts of chelators, you may want to consider a Eu-W8044 chelate. The Eu-W1284-iodoacetamido chelate should be used if you need to label cysteines or other sulfhydryl groups.
Q: What is the difference between ITC- and DTA-activated chelates? They both react with primary amino groups…
A: ITC-activated chelates react with amino and sulfhydryl groups, but the reaction product with sulfhydryl groups decomposes immediately. DTA-activated chelates react with amino groups, sulfhydryl groups (forming a stable conjugate), tyrosine residues, and possibly with some other amino acid residues. DTA-activated chelates are 1.5-2 times more reactive than ITC-activated chelates when proteins are labeled. However, due to the fact that DTA-activated chelates react with several amino acid residues (Lys, Cys, Tyr, amino terminus) on the proteins, there is a danger of proteins becoming inactive upon labeling. ITC-labeling is safer.
Q. What labeling ratio should I aim for?
A. This will depend on the size of the molecule you are labeling. For example, we recommend aiming for 4-10 chelates per protein if the molecular weight of your molecule is greater than 100 kDa. For proteins with a molecular weight in the range of 30-70 kDa, we recommend 2-6 chelates per protein. Proteins with a molecular weight less than 30 kDa should be labeled wtih 1-2 chelates.
Q: Instead of reconstituting the chelate first, can I directly add my protein-to-be-labeled into the vial containing lyophilized chelate?
A: Yes. Reconstituting the chelate in water or sodium acetate allows you to save a portion of unreacted chelate for future use, but you can do your labeling reaction directly in the vial of chelate if your protein is in the correct buffer for labeling (and molar excess chelate over protein/peptide is correct).
Q. What is the molar extinction coefficient for these chelates?
A. For LANCE Eu-W1024, the molar extinction coefficient is 27500 M-1 cm-1 at 335 nm. For LANCE Eu-W8044, the molar extinction coefficient is 34000 M-1 cm-1 at 335 nm.
Q: How should I store my Eu-labeled material?
A: LANCE-labeled reagents must be stored in Tris-HCl buffered solution containing sodium chloride, sodium azide (not necessary when stored at -20°C or -70°C) and BSA (for stabilization). When stored at a reasonable concentration (e.g. no less than 10 µg/mL for antibodies) in this buffer, LANCE-labeled reagents are stable for up to 1-2 years. Please keep in mind the stability is also affected by the stability of the particular reagent being labeled (antibody, peptide), though. We do not recommend storing labeled material in phosphate-based buffers. Labeled proteins and peptides should be stored at a high concentration and in the absence of chelators.
Q: Do I have to label at 4°C?
A: Only peptides (no more than 50 amino acids) can be labeled at room temperature. Temperature is very important when labeling antibodies, which consist of several polypeptide chains. Conditions for antibody labeling should be 4°C, pH 9.3, overnight. If an antibody doesn't stand overnight labeling, then a 4-hour protocol should be followed (using 3 times more chelate than in overnight labeling). Of course, reaction efficiency is higher at RT than at 4°C. However, if a protein loses its binding properties because it has been incubated in the labeling conditions (RT) overnight, then higher labeling efficiency doesn't bring any advantages.
Q: Do I have to label at pH 9.3? My antibody isn't very stable.
A. One possibility to avoid the problem of an overnight reaction at 4°C, pH 9.3, is to perform a 4-hour reaction (4°C, pH 9.3) using 3 times more chelate than in the overnight reaction. The other possibility is to label at pH 8.5-8.6, 4°C, overnight using 4 times more chelate than in the reaction at pH 9.3, 4°C.
Custom labeling and custom assay development at Revvity
Revvity offers custom labeling services as well as custom assay development. If you are interested in having your biomolecule custom-labeled, or in custom assay development, please contact our custom teams:
Custom Labeling and Conjugation Services
Custom Assay Development Services
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























