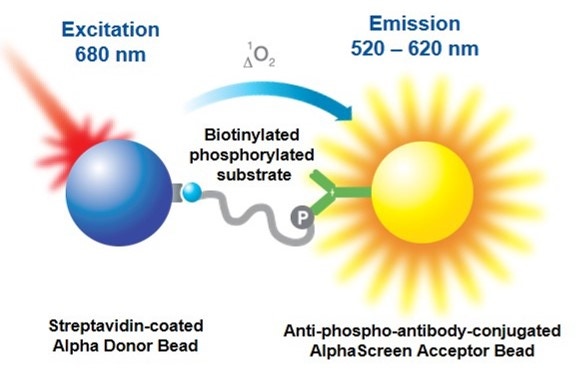
Overview
Alpha kinase assays that employ anti-phospho antibodies can be used in both biochemical and cellular assays. These assays can be developed using one of two formats. The direct format assay will use an anti-phospho antibody that is directly conjugated to an Acceptor bead. The indirect format assay uses an unlabeled anti-phospho antibody that indirectly interacts with a Protein A-coated Acceptor bead or an anti-species Acceptor bead. AlphaLISA™ Acceptor beads should be used when working with complex matrices (such as cell lysates), as they have reduced compound interference compared to AlphaScreen™ Acceptor beads. For this same reason, AlphaLISA beads may be preferred when screening compounds from a library. In either format, your assay is set up so that the substrate will bind the Donor bead, and the Acceptor bead has an anti-phospho antibody that will recognize the phosphorylated motif on the substrate. If your substrate is phosphorylated, the Donor and Acceptor beads are brought together, and excitation of the Donor bead results in the emission of light.

Figure 1. An antibody-based Alpha kinase assay using the direct or indirect format.
Most tyrosine kinase substrates can use a generic anti-phosphotyrosine antibody to recognize the phosphorylated site on the substrate. For serine/threonine kinases, there are some generic antibodies, though it might be easier to use an antibody that is specific to the phosphorylated motif on your substrate.
For the direct format assay, we carry three AlphaScreen anti-phosphotyrosine kits, each of which uses a generic anti-phosphotyrosine antibody (PY20, PT66, or P-Tyr-100) conjugated to the AlphaScreen Acceptor bead. These kits also contain a streptavidin-coated Donor bead and a biotinylated substrate for tyrosine kinases. We also carry unconjugated Acceptor beads, so that you can conjugate your own specific anti-phospho antibody.
For the indirect assay format, we carry Protein A-coated Acceptor beads as well as anti-rabbit, anti-mouse, anti-goat, anti-human, and anti-rat Acceptor beads. You can use these beads to indirectly associate your specific anti-phospho antibody to an Acceptor bead. This format offers some advantages when working with serine/threonine kinases.
What do I need to run this assay?
Required reagents available from Revvity:
- Alpha Donor beads that will bind to your substrate
- AlphaScreen or AlphaLISA Acceptor beads that will be used to recognize the phosphorylated site on your substrate
- TopSeal™-A (plate seal for incubations)
- A microplate (we recommend our light gray AlphaPlates™ or a white plate suitable for fluorescence assays, such as our OptiPlates™ or ProxiPlates™). Also see Microplate selection.
Required reagents available from various suppliers:
- Kinase
- Substrate
- ATP
- EDTA to stop the reaction
- A suitable buffer for your kinase reaction
Instruments/equipment:
- A plate reader (capable of reading Alpha assays)
Protocol-in-brief
As with all assay development, you may need to optimize the protocol for your application.
- Mix the kinase, substrate, and ATP in the well of a 384-well OptiPlate. Incubate at the appropriate temperature for the kinase reaction to proceed.
- Quench the reaction by adding EDTA.
- Add the Donor and Acceptor beads; incubate for 1 hour at room temperature.
- Read plate.
- If you are not sure where to start, we recommend using 20 µg/mL final concentrations of Donor and Acceptor beads, 100 µM ATP, 20 nM kinase (for serine/threonine kinases) or 1 nM kinase (for tyrosine kinases), 50 nM substrate (for biotinylated peptide substrates), and 5 nM anti-phospho antibody (if using an indirect method). All these concentrations will be optimized during assay development.
Assay development
- Titration of enzyme/time-course experiment. This is typically performed as a matrix assay, studying multiple concentrations of kinase at various time points.
- Titration of substrate. For assay sensitivity and linearity, substrate should be used at a concentration equal to or below its Km.
- Titration of antibody (if using indirect format). You may want to test antibody concentrations from 1-30 nM.
- ATP titration. You should test a range of ATP concentrations (usually 10-100 µM) and determine the Km for ATP.
- Inhibition curve. Test an inhibitor of your kinase at a range of concentrations and determine the IC50.
Tips
- If you are using a biotinylated substrate, you will want to titrate the substrate from 1--100 nM. The theoretical maximum capacity of a streptavidin Donor bead is usually around 30 nM (when the beads are used at 20 µg/mL). Please note that this does not take into account the size of the substrate. If your substrate is a large biotinylated protein, you may find you saturate the beads at 10 nM or lower.
- The theoretical maximum capacity of a Protein A Acceptor bead is 3-10 nM when used at 20 µg/mL. Please note that this does not take into account the affinity of your antibody class for Protein A. You may find you saturate the beads at a lower or higher concentration of antibody.
- When titrating, be cautious of using too much substrate or antibody. If you exceed the capacity of the beads, this may result in a hook effect.
- When using tyrosine kinases, remember that generic anti-phosphotyrosine antibodies can recognize not only phosphorylated tyrosines on your substrate, but also phosphorylated tyrosines on your kinase. You may want to keep the concentration of tyrosine kinase in your assay lower than 1 nM.
- If performing a cell-based assay, you may need to transfect your cells so that a tagged version of your substrate will be expressed (this will allow you to use an appropriate affinity bead to capture your substrate). Otherwise, you will need a second antibody that recognizes your "total", unlabeled substrate to create a sandwich assay with the anti-phospho antibody. If using a two-antibody system, you will need to make sure that only one of your antibodies will be recognized by Protein A (when using Protein A beads).
For research use only. Not for use in diagnostic procedures.The information provided above is solely for informational and research purposes only. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.





































