
IVISbrite Shigella dysenteriae Xen27 Bioluminescent Bacterial Strain
IVISbrite Shigella dysenteriae Xen27 Bioluminescent Bacterial Strain
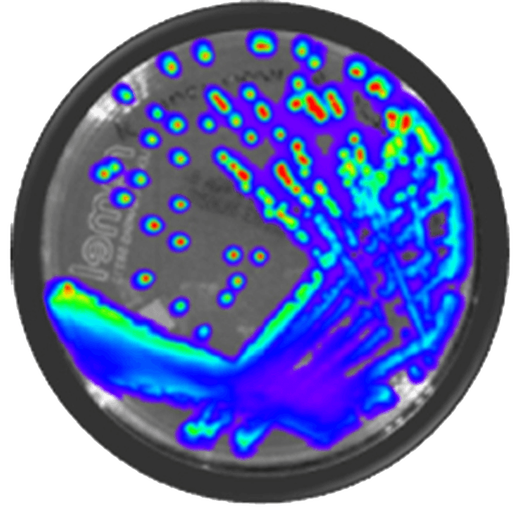


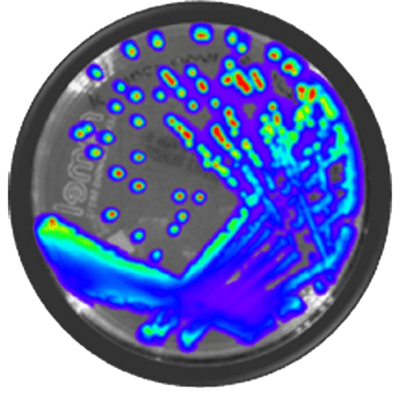
IVISbrite™ Shigella dysenteriae Xen27 bioluminescent bacteria is derived from the 88A6205 clinical isolate. For in vitro or in vivo imaging applications, IVISbrite S. dysenteriae-Xen27 possesses a stable copy of the Photorhabdus luminescens lux operon on the bacterial chromosome.
| Feature | Specification |
|---|---|
| Type | Gram negative |
| Luciferase Classification | Bacterial luciferase (Photorhabdus luminescens lux operon on the bacterial chromosome) |
IVISbrite™ Shigella dysenteriae Xen27 bioluminescent bacteria is derived from the 88A6205 clinical isolate. For in vitro or in vivo imaging applications, IVISbrite S. dysenteriae-Xen27 possesses a stable copy of the Photorhabdus luminescens lux operon on the bacterial chromosome.


IVISbrite Shigella dysenteriae Xen27 Bioluminescent Bacterial Strain


IVISbrite Shigella dysenteriae Xen27 Bioluminescent Bacterial Strain


Product information
Overview
Infectious disease is a perennially important area for both life science research and drug discovery. Some of the first applications of IVIS® imaging technology were in tracking infections and monitoring host responses, and these applications are still at the forefront of research. Our bioluminescent infectious disease light-producing microorganisms have been developed to mimic both acute and chronic standard infection protocols presently used by researchers.
- Engineered Bacterium: Shigella dysenteriae - Xen27
- Parental Strain: 88A6205 clinical isolate
- In Vitro/Ex Vivo: Growth profiles
- In Vivo: Not tested
Advantages of using bioluminescent light-producing microorganisms for drug discovery in vitro:
- Rapid and readily amenable to high throughput screening, e.g., 96 or 384-well format
- Sensitivity - subtle changes in cellular viability are rapidly detected
- Inexpensive - no additional substrates needed for bacterial assays
Advantages of using bioluminescent light-producing microorganisms for drug discovery in vivo:
- Rapid identification of lead drug candidates
- Real-time efficacy data
- May help to decrease drug failure by allowing disease relapse to be monitored in the same animal
Specifications
| Brand |
IVISbrite
|
|---|---|
| Imaging Modality |
Bioluminenscence
|
| Luciferase Classification |
Bacterial luciferase (Photorhabdus luminescens lux operon on the bacterial chromosome)
|
| Shipping Conditions |
Shipped Ambient
|
| Storage Conditions |
-80°C, requires transfer to cryogenic vial prior to storage
|
| Therapeutic Area |
Infectious Diseases
|
| Type |
Gram negative
|
| Unit Size |
1 agar plate
|
Resources
Are you looking for resources, click on the resource type to explore further.
Researchers trust our in vivo imaging solutions to give them reliable, calibrated data that reveals pathway characterization and...
Bacteria growth preparation guidelines using Revvity's IVISbrite™ lux operon labeled bioluminescent bacteria designed for in vivo...
Loading...


How can we help you?
We are here to answer your questions.






























