

HTRF Human Total p53 Detection Kit, 500 Assay Points


HTRF Human Total p53 Detection Kit, 500 Assay Points






The total p53 kit detects cellular p53 and can be used as a normalization assay for the phospho-p53 kit. It is optimal for monitoring cellular response to DNA damage.
| Feature | Specification |
|---|---|
| Application | Cell Signaling |
| Sample Volume | 16 µL |
The total p53 kit detects cellular p53 and can be used as a normalization assay for the phospho-p53 kit. It is optimal for monitoring cellular response to DNA damage.



HTRF Human Total p53 Detection Kit, 500 Assay Points



HTRF Human Total p53 Detection Kit, 500 Assay Points



Product information
Overview
The Total p53 cellular assay kit serves as a normalization assay with our Phospho-p53 kit. P53 has been described as the guardian of the genome because of its role in safeguarding genome integrity. P53 has many anticancer function mechanisms, influencing apoptosis, senescence, cell cycle regulation, growth arrest, genomic stability, angiogenesis inhibition, and metabolism changes, by regulating the expression of various target genes.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Therapeutic Area |
Oncology & Inflammation
|
| Unit Size |
500 assay points
|
Video gallery

HTRF Human Total p53 Detection Kit, 500 Assay Points

HTRF Human Total p53 Detection Kit, 500 Assay Points

How it works
Total-p53 assay principle
The Total-p53 assay quantifies the expression level of p53 in a cell lysate. Contrary to Western Blot, the assay is entirely plate-based and does not require gels, electrophoresis or transfer. The Total-p53 assay uses two labeled antibodies: one coupled to a donor fluorophore, the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of p53 in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the proteins expression under a no-wash assay format.

Total-p53 2-plate assay protocol
The 2 plate protocol involves culturing cells in a 96-well plate before lysis then transferring lysates to a 384-well low volume detection plate before adding Total p53 HTRF detection reagents. This protocol enables the cells' viability and confluence to be monitored.

Total-p53 1-plate assay protocol
Detection of total p53 with HTRF reagents can be performed in a single plate used for culturing, stimulation and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
HTRF assay vs WB using phospho & total P53 assays on human MCF-7 cells
Human MCF-7 cells were plated at 106 cells/ well in a 6 well plate, and incubated for 24h at 37°C, 5% CO2. After treatment for 5 min under UV (2.1J/cm²), the cells were lysed with 200 µL of lysis buffer for 30min at RT under gentle shaking. Serial dilutions of the cell lysate were performed in the supplemented lysis buffer and 16 µL of each dilution were transferred into a 384-well low volume white microplate before the addition of 4 µL of the HTRF phospho-p53 (Ser15) detection reagents. Equal amounts of lysates were used for a side by side comparison of Western Blot and HTRF. Results show that the two HTRF p-53 assays are at least 4-fold more sensitive than the Western Blot.
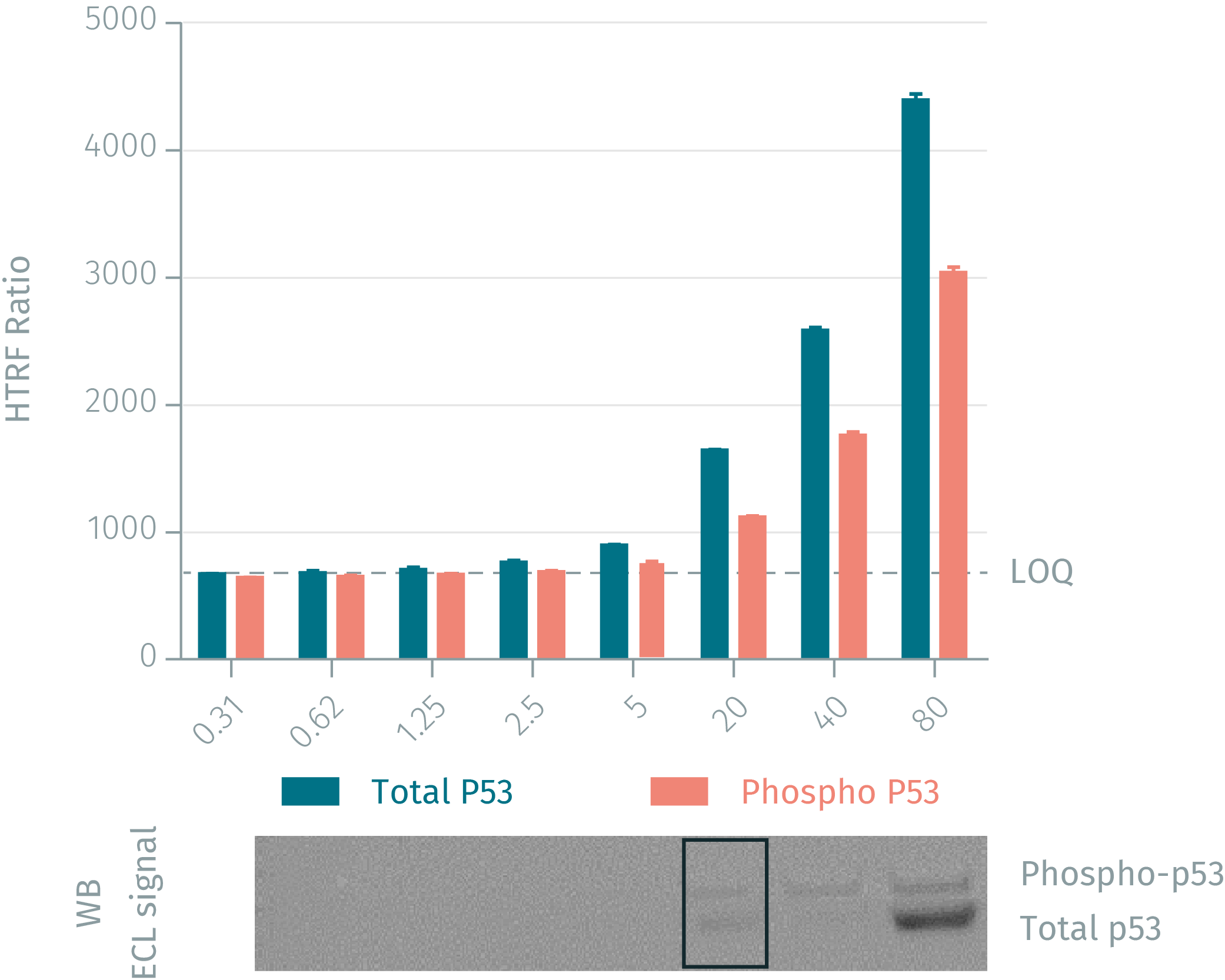
Validation of Total-p53 assay on Neocarcinostatin treated MCF-7 breast cancer cells
Human MCF-7 cells were plated at 100,000 cells/ well in a 96 well plate, and incubated for 24h at 37°C, 5% CO2. After treatment for 45 min with increasing concentrations of Neocarcinostatin, the medium was removed and the cells were lysed with 50 µL of lysis buffer for 30min at RT under gentle shaking. 16 µL of lysate were transferred into a 384-well low volume white microplate and 4 µL of the HTRF phospho-p53 (Ser15) or total p53 detection reagents were added. The HTRF signal was recorded after an overnight incubation.

Validation of Total-p53 assay Etoposide (apoptosis inductor) treated breast cancer MCF-7 cells
Human MCF-7 cells were plated at 200,000 cells/ well in a 96 well plate, and incubated for 24h at 37°C, 5% CO2. After treatment for 3 h with increasing concentrations of Etoposide, the medium was then removed and the cells were lysed with 50 µL of lysis buffer for 30min at RT under gentle shaking. 16 µL of lysate were transferred into a 384-well low volume white microplate and 4 µL of the HTRF phospho-p53 (Ser15) or total p53 detection reagents were added. The HTRF signal was recorded after an overnight incubation.

Simplified pathway
Function and regulation of p53
P53 becomes activated by a myriad of stressors including, but not limited to, DNA damage, oxidative stress, osmotic shock, ribonucleotide depletion, and deregulated oncogene expression. In response to such stresses, p53 undergoes extensive posttranslational modifications, including phosphorylation and acetylation. Phosphorylation of p53 mostly occurs in the N-terminal at Ser15 and leads to a reduced interaction between p53 and its negative regulator, the oncoprotein MDM2, which inhibits p53 accumulation by targeting it for ubiquitination and proteasomal degradation. Phosphorylation in this site impairs the ability of MDM2 to bind p53, promoting both the accumulation and activation of p53 in response to DNA damage.

Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...
This guide provides you an overview of HTRF applications in several therapeutic areas.
An in-depth review of molecular and cellular pathways
The maintenance of proteostasis, the biological mechanisms that control the...
SDS, COAs, Manuals and more
Are you looking for technical documents related to the product? We have categorized them in dedicated sections below. Explore now.
- LanguageEnglishCountryUnited States
- LanguageFrenchCountryFrance
- LanguageGermanCountryGermany
- Lot Number04JLot DateApril 3, 2026
- Lot Number04GLot DateApril 3, 2026
- Lot Number04HLot DateApril 3, 2026
- Resource TypeManualLanguageEnglishCountry-


How can we help you?
We are here to answer your questions.






























